Determining the lipid components of wheat germ by nuclear magnetic resonance
-
摘要:
为深入探究小麦胚芽中的脂质成分,该研究采用不同溶剂分别提取小麦胚芽中的磷脂和中性脂质,利用核磁共振技术 (nuclear magnetic resonance,NMR)对小麦胚芽中的脂质成分进行分析,结果表明:31P NMR在小麦胚芽中检测到6种磷脂和相对应的5种溶血性磷脂,以及非脂质含磷化合物甘油磷脂酰胆碱(GPC);磷脂中含量最高的是磷脂酰胆碱(PC),其摩尔浓度为0.42 μmol/g,摩尔分数为28.50%,质量浓度为0.31 mg/g,质量分数为30.20%;1H NMR测定小麦胚芽中的多种中性脂质组成及含量,包括甘油三酯(TG)、甘油二酯(DG)、甘油单酯(MG)和游离脂肪酸(FA),其中含量最高的甘油酯是TG,占比为77.25%,含量最低的是2-甘油单酯(2-MG),占比为0.03%;小麦胚芽的甘油三酯和磷脂中检测出亚油酸(L)、油酸(O)和亚麻酸(Ln)等6种不饱和脂肪酸,其中亚油酸的含量最高,在甘油三酯中占比56.26%,在磷脂中占比45.37%。溶血性磷脂和GPC是磷脂的水解产物,DG、MG和FA是甘油三酯的水解产物,这些物质可以反映样品中脂质的水解程度。研究结果表明,利用NMR不仅能够对小麦胚芽脂质的组分进行定性定量分析,而且可以监测小麦胚芽脂质水解程度的变化。NMR技术在小麦胚芽相关产品的脂质分析研究具有重要作用。
Abstract:Wheat germ is rich in lipids with a very complex composition. The lipids in wheat germ are prone to be hydrolyzed and oxidized during processing and storage. In this study, phospholipids and neutral lipids were extracted from wheat germ with different solvents. Then the phospholipids, mono-, di-, and triacylglycerols, as well as fatty acyl group contents were analyzed by nuclear magnetic resonance (NMR) spectroscopy. The results showed that six kinds of phospholipids were detected by 31P NMR in wheat germ, including phosphatidylcholine (PC), phosphatidyl ethanolamine (PE), phosphatidyl inositol (PI), phosphatidylglycerol (PG), phosphatidic acid (PA), and phosphatidylserine (PS). Furthermore. There were also five kinds of lysophospholipids (lysophosphatidylcholine (LPC), lysophosphatidylglycerol (LPG), and lysophosphatidylethanolamine (LPE)) and two non-lipid phosphorus compounds (inorganic phosphate (pi) and phosphatidylcholine glycerol (GPC)). GPC was the hydrolytic product from the removal of fatty acyl groups by two-step hydrolysis of PC. PC had the highest content of phospholipid in the wheat germ. Specifically, the molar concentration of PC was 0.42 μmol/g wheat germ, the molar fraction of PC was 28.50 %, the mass concentration of PC was 0.31 mg/g wheat germ, and the mass fraction of PC was 30.20 %. The signals of PE, PC, LPC, GPC, and choline (Cho) in 1H NMR spectra were also used to determine the phospholipids in wheat germ. Compared with 1H NMR, 31P NMR spectra were better resolved and more suitable for qualitative and quantitative analysis of phospholipid components, as 31P NMR only determined the characteristic signal of phosphorus-containing compounds. The composition and content of free fatty acids, mono-, di-, and triacylglycerols were determined in the wheat germ by 1H NMR. The proportion of free fatty acids was 12.35 % in the wheat germ. The highest content was triacylglyceride (TG), accounting for 77.25 %. The contents of 1,3-diacylglycerol (1,3-DG) and 1, 2-diacylglycerol (1,2-DG) were 5.80 % and 4.41 %, respectively, and the contents of 1-monoacylglycerol (1-MG) and 2-monoacylglycerol (2-MG) were 0.17% and 0.03%, respectively. Moreover, TG was gradually hydrolyzed to produce diglycerol (DG), monoglycerol (MG), free fatty acid (FA) and glycerol in the wheat germ during storage. FA was always produced in the wheat germ throughout the hydrolysis reaction, leading to a higher content, compared with DG and MG. Six kinds of fatty acyl groups were detected by 1H NMR in triglycerides and phospholipids of wheat germ, including the acyl groups of docosahexaenoic acid (DHA), eicosapentaenoic acid and arachidonic acid (EPA+ARA), linolenic acid (Ln), linoleic acid (L) and oleic acid (O). Linoleic acid was the most abundant unsaturated fatty acyl group in triglycerides and phospholipids. Saturated and modified acyl groups (S+M) were also detected by 1H NMR. There was significant difference in fatty acid composition between triglyceride and phospholipid in wheat germ. Lysophospholipids and GPC were the hydrolyzed products of phospholipids, while DG and MG were the hydrolyzed products of triglycerides. As such, their contents shared the degree of lipids hydrolysis in the wheat germ. Therefore, NMR can be used to determine the lipid compositions and hydrolysis in the wheat germ. In short, this NMR is a powerful tool for lipids analysis of wheat germ products.
-
Keywords:
- lipid /
- phospholipids /
- wheat germ /
- 31P nuclear magnetic resonance /
- 1H nuclear magnetic resonance
-
0. 引 言
小麦胚芽又称麦芽粉、胚芽,金黄色颗粒状,是小麦发芽及生长的器官,约占麦粒质量的2%~3%,具有极高的营养价值[1]。小麦胚芽中脂肪质量分数约为11%,其中不饱和脂肪酸占比超过80%,主要包括亚油酸、油酸和亚麻酸;饱和脂肪酸主要包括棕榈酸和硬脂酸;小麦胚芽中还含有少量磷脂(0.13%~0.18%)[2-4]。富含营养的小麦胚芽可广泛应用于食品、药品和化妆品等领域。
核磁共振技术(nuclear magnetic resonance,NMR)是一种基于原子核磁性的分析技术,指的是具有自旋性的原子核,在恒定磁场与交变磁场共同作用下,原子核与电磁波相互作用,发生能级跃迁,产生共振信号,从而通过样品的化学位移、官能团、信号面积等参数信息对样品进行定性和定量分析[5]。核磁共振技术是通过比率测量的方法,使用内标即可定量,具有对样品结构性质无损伤性、易于量化、几乎不需要分离、允许识别新化合物并且不需要化学衍生等优势,并具有高准确度和重复性的特点[6-7]。目前大多数脂质的NMR研究是在采用有机溶剂提取待测样品之后进行的,在该过程消除了其他有机化合物(如碳水化合物和蛋白质)的干扰。
LI等[8]的研究表明核磁共振可根据不同代谢物在单个原子的化学结构和化学环境上的差异提供独特的指纹图谱,在脂质的分离提取后,利用NMR技术通过化学位移比对,可清晰地将高度不饱和的脂肪酰基残基与其他脂肪酰基残基区分开,从而不仅能够鉴定烯烃、胆碱上的甲基、烷烃和ω-3脂肪酰基残基等官能团,还可识别出不同类型的脂质(如胆固醇、甘油三酯和磷脂),并结合磷脂和中性脂质的1H NMR分析结果,可得到整个脂质组学的图谱。YANG等[9]利用1H NMR技术对蛋黄磷脂脂质体测定得到了脂肪酰基组成。WAN等[10]采用核磁共振技术监测鱼头汤烹饪过程中磷脂以及相关氧化产物的演变。叶婷婷等[11]利用1H NMR技术对奶油中极性与非极性物质进行定性定量分析,检测到氨基酸、还原糖、饱和脂肪酸、双甘酯和单甘酯等物质成分。
由于小麦胚芽中含有丰富的不饱和脂肪酸和多种高活性酶,在贮藏期间极易发生水解和酸败变质情况,制约了小麦胚芽精深加工产业的发展。与色谱技术相比,核磁共振技术具有对待测化合物的纯度要求低、前处理简单,不需要参考目标物质标准品进行定性定量,一种内标物便可定量分析多种物质或多组分物质等优势,能够简单高效地提供样品中存在的各种化合物的详细信息,因此推测NMR技术对小麦胚芽脂质研究可起到重要作用[12-15]。本研究利用31P NMR技术分析磷脂组成,同时利用1H NMR技术测定甘油单酯(MG)、甘油二酯(DG)、甘油三酯(TG)和游离脂肪酸(FA)的含量以及脂肪酸组成,以期为小麦胚芽的开发利用和其他粮食含量监测研究提供理论依据和技术参考。
1. 材料与方法
1.1 材料与试剂
新鲜小麦胚芽由桂林智仁食品工业有限公司赠送提供。
胆酸钠、草甘膦、叔丁基对苯二酚(TBHQ)、氘代水(99.9%,D2O)、氘代氯仿(99.8% CDCl3+0.03% TMS),上海麦克林生化科技有限公司;乙醇、丙酮,成都市科隆化学品有限公司;正己烷,天津市大茂化学试剂厂;二氯甲烷,天津奥普升化工有限公司;乙二胺四乙酸,天津博迪化工股份有限公司;氢氧化钾,重庆川东化工有限公司。
1.2 仪器与设备
PL203型电子分析天平,梅特勒-托利多仪器(上海)有限公司;XT-350多功能粉碎机,永康市红太阳机电有限公司;T 25 digital分散机,艾卡(广州)仪器设备有限公司;TD5A-WS台式低速离心机,湖南湘仪实验室仪器开发有限公司;HJ-6A数显恒温磁力搅拌器,江苏金怡仪器科技有限公司;RE-201D旋转蒸发仪,上海精密仪器仪表有限公司;GE001 Mini 迷你涡旋混匀仪,武汉君诺德生物技术有限公司;AVANCE III HD 500 NMR波谱仪,德国Bruker公司。
1.3 方 法
1.3.1 样品的制备
将新鲜小麦胚芽用多功能粉碎机磨成粉,过20目(孔径为0.85 mm)筛后,将小麦胚芽粉密封存放至-20 ℃冰箱中备用。每组测定样品皆取自同一批小麦胚芽。
1) 小麦胚芽磷脂粗提物的制备
参考AHMMED等[16-17]的方法并稍作调整。称量30 g小麦胚芽样品至500 mL烧杯中,加入200 mL乙醇(95%,体积分数)和100 mL正己烷(99%,体积分数),在烧杯外壁包裹冰的条件下进行均质(15 000 r/min,5 min),匀浆离心(4 ℃,2 000 r/min,15 min)后抽滤提取滤液。麦胚残渣用乙醇与己烷的混合溶剂(2 : 1)重复提取2次,合并滤液,减压蒸发溶剂后备用。利用-20 ℃的丙酮沉淀磷脂,再多次洗涤至溶液呈无色,真空下去除溶剂(10 h),得到小麦胚芽磷脂粗提物。
2) 小麦胚芽中性脂质的制备
参考NIEVA-ECHEVARRÍA等[18]的方法。称取20 g小麦胚芽,加入40 mL二氯甲烷作为提取溶剂,在室温下搅拌30 min后,抽滤取滤液,用二氯甲烷重复提取2次。将收集的滤液混合,在减压条件下用旋转蒸发仪除去二氯甲烷后,提取物在室温下真空处理10 h,用于小麦胚芽的甘油单酯、甘油二酯、甘油三酯和游离脂肪酸的测定。
参考ZHAO等[17]的方法并稍作调整。称取20 g小麦胚芽,加入100 mL正己烷和0.4 mg TBHQ混合搅拌30 min,抽滤取滤液,用正己烷重复提取2次。收集滤液至分液漏斗中,用90%乙醇洗涤至乙醇层无色,以除去极性脂质杂质。取正己烷溶液层在减压条件下旋转蒸发溶剂后,在室温下真空处理10 h,提取物用于小麦胚芽中甘油三酯脂肪酰基组成的测定。
1.3.2 31P NMR分析
参考AHMMED等[19]的方法并稍作调整。配制含有20%(体积分数)D2O、10%(g/ML)胆酸钠、1%(g/ML)乙二胺四乙酸和0.846 μmol/mL草甘膦的混合溶剂,用1 mol/L的KOH将pH值调节至7.4~7.8。将0.1 g小麦胚芽磷脂粗提物溶解在0.6 mL混合溶剂中,对溶液进行涡旋处理10 min,将混合物以10 000 r/min离心10 min,将透明溶液转移至5 mm NMR管中进行分析。草甘膦(GLP)作为D2O混合溶剂中定量磷脂化合物的内标物,基于先前的文献报道[16, 19]获得磷脂31P NMR光谱的化学位移,以磷脂酰胆碱(PC,化学位移δ为-0.87)作为化学位移参考。对样品分别进行4次31P NMR分析。31P NMR测定参数:温度25℃;扫描次数192;弛豫时间3.5 s。
运用MESTRENOVA(12.0.0)对核磁光谱进行处理,对化合物进行定性和定量。定性是利用待测化合物官能团的化学位移和峰型,与核磁光谱信号峰对照进行比对判断。定量是通过选择远离拥挤的光谱区域且不与待测化合物信号重叠的内标化合物,对内标和待测化合物的特征信号峰进行积分获得峰面积,利用信号峰面积与样品中存在的质子数成正比的原理,通过比较内标和待测化合物特征信号峰面积计算待测化合物的含量[7]。
磷脂组分的摩尔浓度通过式(1)和式(2)计算[17]:
ai=Ai⋅cGLP⋅vAGLP⋅m×106 (1) xi=ai∑ai×100% (2) 式中ai为待测磷脂的摩尔浓度,μmol/g;Ai为待测磷脂的积分值;cGLP为草甘膦的摩尔浓度,0.000 846 mol/L;v为所取D2O混合溶剂的体积,0.000 6 L;AGLP为草甘膦的积分值;m为制备0.1 g小麦胚芽磷脂粗提物所需的小麦胚芽质量,2.380 5 g;106为换算系数;xi为待测磷脂的摩尔分数,%;Σai为各磷脂组分的摩尔浓度之和,μmol/g。
磷脂组分的质量浓度和质量分数则通过各磷脂的摩尔浓度和相对分子质量进行计算。由于磷脂的甘油骨架sn-3位结合了磷酸基团,而sn-1和sn-2位会结合不同脂肪酰基[20]。在本研究中相对分子质量的选取参考小麦胚芽或小麦相关文献[21-23]报道中含量最高的目标磷脂脂肪酰基组合,并与数据库(https://hmdb.ca/)比对,将相关信息整理为表1。磷脂组分的质量浓度和质量分数通过式(3)和式(4)计算:
表 1 小麦或小麦胚芽中含量最高的含磷化合物的分子信息Table 1. Molecular information on the most abundant phosphorus-containing compounds in wheat or wheat germ含磷化合物
Phosphorous-containing compound脂肪酰基组成
Fatty acyl groups composition化学分子式
Chemical formula相对分子质量
Relative molecular massPC 棕榈酸/亚油酸(16:0/18:2) C42H80NO8P 742.061 PI 棕榈酸/亚油酸(16:0/18:2) C43H79O13P 835.066 LPC 亚油酸(18:2) C26H50NO7P 519.660 PS 油酸/亚油酸(18:1/18:2) C42H76NO10P 786.027 PE 亚油酸/亚油酸(18:2/18:2) C41H74NO8P 740.002 GPC - C8H20NO6P 257.223 LPE 亚油酸(18:2) C23H44NO7P 477.572 PG 棕榈酸/亚油酸(16:0/18:2) C40H75O10P 746.991 LPG 棕榈酸(16:0) C22H45O9P 484.567 pi - PO43- 94.973 PA 亚油酸/亚油酸(18:2/18:2) C39H69O8P 696.947 注:PC为磷脂酰胆碱;PI为磷脂酰肌醇;LPC为溶血磷脂酰胆碱;PS为磷脂酰丝氨酸;PE为磷脂酰乙醇胺;GPC为甘油磷脂酰胆碱;LPE为溶血磷脂酰乙醇胺;PG为磷脂酰甘油;LPG为溶血磷脂酰甘油;pi为无机磷酸盐;PA为磷脂酸,下同。磷脂的相对分子质量参考文献[21-23];无机磷酸盐(pi)的相对分子质量依据磷酸根进行计算;甘油磷脂酰胆碱(GPC)和pi为非脂质含磷化合物,不属于磷脂,则不计算其质量分数。 Note: PC is phosphatidylcholine; PI is phosphatidylinositol; LPC is lysophosphatidylcholine; PS is phosphatidylserine; PE is phosphatidylethanolamine; GPC is glycerophosphocholine; LPE is lysophosphatidylethanolamine; PG is glycerophosphoglycerol; LPG is lysophosphatidylglycerol; pi is inorganic phosphate; PA is phospholipid acid,the same below. The relative molecular weight of phospholipids refers to relevant literature[21-23]; The relative molecular mass of inorganic phosphate (pi) is calculated according to phosphate group; Glycerol phosphatidylcholine (GPC) and pi are non-lipid phosphatide-containing compounds and do not belong to phospholipids, so their mass fractions are not calculated. bi=ai×Mi×10−3 (3) yi=bi∑bi×100% (4) 式中bi为待测磷脂的质量浓度,mg/g;ai为待测磷脂的摩尔浓度,μmol/g;Mi为待测磷脂的相对分子质量;10−3为换算系数。yi为待测磷脂的质量分数,%;Σbi为各磷脂组分的质量浓度之和,mg/g。
1.3.3 1H NMR分析
1) 利用 D2O混合溶剂测定
将0.1 g小麦胚芽磷脂粗提物溶解在0.6 mL D2O混合溶剂中,处理过程同章节1.3.2。将溶液转移至5 mm核磁管中,用500 MHz光谱仪进行测定。1H NMR测定参数[17]:弛豫时间3 s;扫描次数128次。基于先前的文献[24-26]报道获得磷脂1H NMR光谱的化学位移,其中以D2O(δ为4.79)作为参考,对样品分别进行3次1H NMR分析。
2) 利用CDCl3溶剂测定
小麦胚芽磷脂脂肪酰基的测定参考ZHAO等[17]的方法并稍作调整。将0.1 g小麦胚芽磷脂粗提物溶解在0.8 mL CDCl3(含有0.03% TMS)溶剂中,再加入0.8 mL的乙二胺四乙酸溶液(0.2 mol/L,pH值为 7.2~7.5),溶液涡旋5 min后,在6 000 r/min条件下离心10 min,再将0.6 mL底部有机层转移到5 mm核磁共振管中。中性脂质的测定参考楼乔明等[27]的方法并稍作调整。将利用二氯甲烷和正己烷分别提取得到的两组中性脂质样品各称取0.1 g溶解在0.6 mL CDCl3(含有0.03% TMS)溶剂中,溶液涡旋5 min。将溶液转移至5 mm核磁管中,用500 MHz光谱仪进行测定。1H NMR测定参数:弛豫时间3 s;扫描次数128次。四甲基硅烷(TMS)作为CDCl3溶剂中的内标物,化学位移以TMS(δ为0)作为参考,化学位移分布见表2。对两组样品分别进行3次1H NMR分析。
表 2 主要脂肪酰基在CDCl3中1H NMR信号的化学位移分配和多重峰种类Table 2. Chemical shift assignments and multiplicities of the 1H NMR signals in CDCl3 of the main acyl groups信号
Signal化学位移
Chemical shift δ峰型
Multiplicity官能团
Functional group组分
Compound参考文献
ReferenceA 0.83~0.93 m -CH3 饱和、单不饱和ω-9和/或ω-7、不饱和ω-6酰基 [28] B 0.97 t -CH3 不饱和ω-3酰基 [28] C 1.66~1.70 m -OCO-CH2-CH2- EPA和ARA酰基 [17] D 1.94~2.14 m -CH2-CH=CH- 除DHA外的脂肪酸烯丙基 [28] E 2.26~2.36 dt -OCO-CH2- 除DHA外的脂肪酰基 [18] F 2.36~2.42 m -OCO-CH2-CH2- DHA酰基 [28] G 2.76 t =HC-CH2-CH= 双不饱和ω-6酰基 [28] H 3.65 ddd ROCH2–CHOH–CH2OH 1-MG中的甘油基 [18] I 3.73 m ROCH2–CH(OR′)–CH2OH 1,2-DG中的甘油基 [18] J 3.84 m ROCH2–CH(OR′)–CH2OH 2-MG中的甘油基 [18] K 3.94 m ROCH2–CHOH–CH2OH 1-MG中的甘油基 [18] L 4.05~4.10 m ROCH2–CHOH–CH2OR′ 1,3-DG中的甘油基 [18, 29] M 4.14 dd -CH2OCOR TG中的甘油基 [17] N 4.18 ddd ROCH2–CHOH–CH2OH 1-MG中的甘油基 [18] O 4.30 dd -CH2OCOR TG中的甘油基 [17] P 4.32~4.38 ddd ROCH2–CH(OR')–CH2OH 1,2-DG中的甘油基 [18, 29] 注:信号与图2、图3中信号峰上标相对应。 Note: The signal corresponds to the superscript of the signal peak in figure 2 and figure 3. 不同甘油结构(MG、DG、TG)和FA的酰基的摩尔数和摩尔百分比的计算方法如表3所示,比例常数PC通过式(5)计算[17-18]:
表 3 不同甘油结构(MG、DG、TG)和FA的酰基的摩尔数(N)和摩尔百分比(AG)的计算式Table 3. Calculation formula of the number of moles(N) and molar percentages of acyl groups (AG) supported on the different glyceride structures (MG, DG, TG) and fatty acid( FA)脂肪组分
Lipid component摩尔数计算式
Molar number calculation formula摩尔百分比计算式
Molar percentage calculation formula1-MG N1-MG=PC⋅AK AG1-MG=N1-MGNT×100\text{%} 2-MG N2-MG=PC×AJ4 AG2-MG=N2-MGNT×100\text{%} 1,2-DG N1,2-DG=PC×(AH+I-2AK)2 AG1,2-DG=2N1,2-DGNT×100\text{%} 1,3-DG N1,3-DG=PC×(AL+M+N+O+P−2AK)5 AG1,3-DG=2N1,3-DGNT×100\text{%} TG NTG=PC×(2AO+P-AH+I+2AK)4 AGTG=3NTGNT×100\text{%} FA NFA=PC×AE+F-6NTG-4N1,2-DG−4N1,3-DG−2N1-MG−2N2-MG2 FA=NFANT×100\text{%} 注:1-MG为1-甘油单酯;2-MG为2-甘油单酯;1,2-DG为1,2-甘油二酯;1,3-DG为1,2-甘油二酯;TG为甘油三酯;FA为游离脂肪酸;Pc 是1H NMR波谱信号区与产生这些信号区的质子数之间的比例常数,15.84,计算方法为式(5);AK,AJ,AH+I,AL+M+N+O+P,AO+P和AE+F表示1H NMR波谱信号,与表2、图2信号峰上标相对应;该计算不考虑微量磷脂的影响[18]。 Note:1-MG is 1-monoacylglycerol; 2-MG is 2-monoacylglycerol; 1,2-DG is 1,2-diacylglycerol; 1,3-DG is 1,3-diacylglycerol; TG is triglyceride; FA is free fatty acid; Pc is the proportional constant between the signal region of 1H NMR spectrum and the number of protons generating these signal regions, Pc is 15.84, and the calculation method is shown in equation (5); AK, AJ, AH+I, AL+M+N+O+P, AO+P and AE+F represent 1H NMR spectral signals, corresponding to the superscript of signal peaks in table 2 and figure 2; This calculation does not take into account the effect of trace phospholipids[18]. PC=nTMSATMS12×106 (5) 式中PC为比例常数;nTMS为内标TMS物质的量,1.32×10−6 mol;ATMS为内标TMS在1H NMR谱图中的积分;12为内标TMS的氢原子个数;106为转换系数。
不同甘油结构(MG、DG、TG)和FA的酰基的总摩尔数通过式(6)计算[18]:
NT=3NTG+2N1,2−DG+2N1,3−DG+N1−MG+N2−MG+NFA (6) 式中NT为样品中存在的酰基与FA的总摩尔数,μmol;N是相应化合物的摩尔数,μmol。
脂肪酸组成可以通过每个脂肪酰基链的特征信号强度与来自甘油主链之一的特征信号强度之间的关系来确定。甘油三酯和磷脂中的主要脂肪酰基的积分计算公式如表4所示,主要脂肪酰基的摩尔百分比通过式(7)计算[17]:
表 4 脂肪酰基的积分计算公式Table 4. Integral calculation formula of fatty acyl groups脂肪酰基
Lipid acyl group积分计算公式
Integral formulaLn ILn=AB3 EPA+ARA IEPA+ARA=AC2 DHA IDHA=AF4 L IL=AG2 O I(TG-O)=AD4-ILn-IL-IEPA+ARA I(PL-O)=AD4-(ILn-IDHA)2-IL-IEPA+ARA S+M IS+M=IFA-AD4-IDHA FA I(TG-FA)=AM+O4×3 I(PL-FA)=AA+B3 注:Ln为亚麻酸;EPA+ARA为二十碳五烯酸与花生四烯酸;DHA为二十二碳六烯酸;L为亚油酸;O为油酸;S+M为饱和脂肪酸与修饰酰基;FA为总脂肪酸;TG-O和TG-FA是指甘油三酯油酸和总脂肪酸,PL-O和PL-FA是指磷脂的油酸和总脂肪酸[17];AB,AC,AF,AG,AD,AM+O和AA+B表示1H NMR波谱信号,与表2、图3相对应。 Note: Ln is linolenic acid; EPA+ARA is eicosapentaenoic acid and arachidonic acid; DHA is docosahexaenoic acid; L is linoleic acid; O is oleic acid; S+M is saturated fatty acid and modified acyl group; FA is total fatty acids; TG-O and TG-FA refer to triglyceride oleic acid and total fatty acid, and PL-O and PL-FA refer to oleic acid and total fatty acid of phospholipids[17]; AB, AC, AF, AG, AD, AM+O and AA+B represent 1H NMR spectral signals, corresponding to the superscript of signal peaks in table 2 and figure 3. zi=IiITG−FA=IiIPEFA×100% (7) 式中zi为待测脂肪酸的摩尔百分比,%;Ii为待测脂肪酸的积分换算值;ITG-FA为甘油三酯总脂肪酸的积分换算值;IPL-FA为磷脂总脂肪酸的积分换算值。
1.4 数据处理
运用SPSS 26.0软件进行数据分析,并用Origin 2021软件进行绘制图形。
2. 结果与分析
2.1 小麦胚芽磷脂的NMR分析
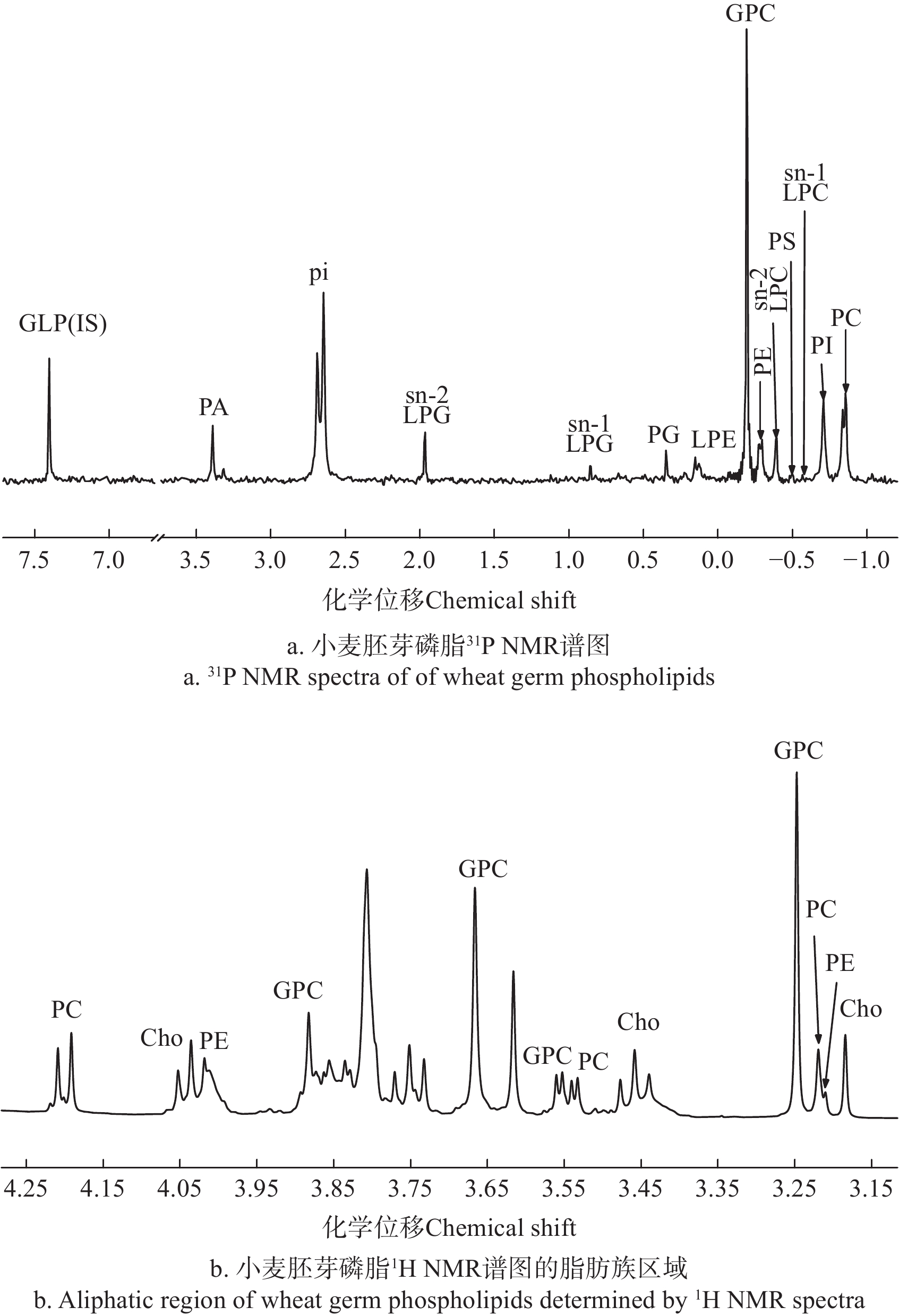
磷脂在生物膜的构成中起到重要作用。为获得新鲜小麦胚芽磷脂组分及其含量,采用31P NMR和1H NMR对样品进行分析,结果如表5和图1所示。
表 5 利用31P NMR测定小麦胚芽含磷化合物的摩尔浓度、摩尔分数、质量浓度和质量分数Table 5. Molar concentration, molar fraction, mass concentration and mass fraction of phosphorus-containing compounds in wheat germ were determined by 31P NMR含磷化合物
Phosphorous-containing compound化学位移
Chemical shift (δ)摩尔浓度
Molar concentration/(μmol·g−1)摩尔分数
Molar fraction/%质量浓度
Mass concentration/(mg·g−1)质量分数
Mass fraction/%PC -0.87 0.42±0.03 28.50±1.33 0.31±0.03 30.20±1.46 PI -0.72 0.29±0.01 19.87±1.24 0.24±0.01 23.70±1.46 sn-1 LPC -0.58 0.04±0.01 2.48±0.83 0.02±0.01 1.84±0.64 PS -0.51 0.05±0.04 3.25±2.47 0.04±0.03 3.62±2.73 sn-2 LPC -0.40 0.11±0.01 7.64±0.49 0.06±0.00 5.67±0.39 PE -0.30 0.19±0.01 13.20±1.01 0.14±0.01 13.96±1.12 GPC -0.20 0.75±0.10 - 0.19±0.03 - LPE 0.13 0.06±0.02 4.37±1.56 0.03±0.01 2.98±1.08 PG 0.34 0.05±0.01 3.31±0.65 0.04±0.01 3.52±0.67 sn-1 LPG 0.84 0.03±0.01 2.01±0.63 0.01±0.00 1.39±0.44 sn-2 LPG 1.95 0.11±0.02 7.61±1.51 0.05±0.01 5.26±1.05 pi 2.65 1.00±0.14 - 0.09±0.01 - PA 3.34 0.16±0.02 10.63±1.09 0.11±0.01 10.58±1.08 TP - 1.46±0.06 - 1.02±0.04 - TPC - 3.21±0.22 - 1.31±0.06 - 注: sn-1 LPC为sn-1溶血磷脂酰胆碱; sn-2 LPC为sn-2溶血磷脂酰胆碱; sn-1 LPG为sn-1溶血磷脂酰甘油;sn-2 LPG为sn-2溶血磷脂酰甘油;TP为总磷脂;TPC为总含磷化合物。含磷化合物缩写与图1信号峰上标相对应,摩尔分数和质量分数指的是各磷脂在总磷脂中所占百分比,甘油磷脂酰胆碱(GPC)和无机磷酸盐(pi)不属于磷脂,则不计算其摩尔分数和质量分数。 Note: sn-1 LPC is sn-1 lysophosphatidylcholine; sn-2 LPC is sn-2 lysophosphatidylcholine; sn-1 LPG is sn-1 lysophosphatidylglycerol; sn-2 LPG is sn-2 lysophosphatidylglycerol; TP is total phospholipids; TPC is total phosphorus compounds. The abbreviations of phosphorus-containing compounds correspond to the superscript of the signal peak in figure 1, The molar fraction and mass fraction are the percentage of each phospholipid in the total phospholipid, glycerol phosphatidylcholine (GPC) and inorganic phosphate (pi) are not phospholipids, and their molar fraction and mass fraction are not calculated. 由表5可知,小麦胚芽中共鉴定出了13种含磷化合物。这些含磷化合物在相同的NMR条件下都具有相同的化学位移,因此很多相关研究并不需要这些化合物标准品,而是根据其化学位移直接进行定性分析[30-31]。与参考文献[23, 32]利用色谱技术分析结果一致,利用31P NMR可以检测出小麦籽粒中常见且含量较高的磷脂,例如磷脂酰胆碱(PC)、磷脂酰乙醇胺(PE)、sn-2溶血磷脂酰胆碱(sn-2 LPC)、溶血磷脂酰乙醇胺(LPE)、磷脂酰肌醇(PI)、磷脂酸(PA)、磷脂酰甘油(PG)等。此外,利用31P NMR技术还能够对小麦胚芽中的无机磷酸盐(pi)和GPC,以及包括sn-1溶血磷脂酰甘油(sn-1 LPG)、sn-2溶血磷脂酰甘油(sn-2 LPG)和sn-1溶血磷脂酰胆碱(sn-1 LPC)的溶血性磷脂进行有效的定性定量分析。其中由于GPC和鞘磷脂(SM)在31P NMR谱图中的化学位移仅相差0.03[33],且PELILLO等[23]利用高效液相色谱-蒸发光散射检测器(HPLC-ELSD)测定小麦磷脂组成的研究中未检测到SM,则本研究考虑将小麦胚芽31P NMR光谱中化学位移-0.20的单峰定性为GPC的信号峰。值得一提的是,31P NMR技术可以检测到含量低的sn-1 LPC、sn-1 LPG等溶血性磷脂异构体,其形成原因是溶血性磷脂中具有游离羟基的sn-2位参与了分子内酰基迁移反应,酰基残基从sn-2向sn-1位迁移[34-35],该结果体现了31P NMR技术的优势。
本试验测定出小麦胚芽的总磷脂质量浓度为1.02 mg/g,低于部分文献所示值。MORUZZI等[3]采用柱色谱法测定得到磷脂质量浓度为1.30 mg/g,XU等[4]采用钼蓝比色法测定得到磷脂质量浓度为1.45~1.81 mg/g,两者都是通过测定油脂样品中的磷含量,计算(磷含量×换算系数)转换得到的磷脂质量,其缺点是当磷脂提取物中含有其它含磷化合物杂质(如无机磷酸盐)时,计算得到的磷脂质量将会偏大,准确性较低。本研究利用31P NMR技术测定磷脂含量不受样品纯度的影响,可获取小麦胚芽磷脂粗提物中所有含磷化合物的详细信息,在计算和分析过程中不会受到杂质的干扰,得到更准确的数据结果。除了分析定量方法的差异之外,胚芽从小麦中提取的工艺和水分含量的不同也可能导致测定得到的小麦胚芽磷脂含量存在差异。本试验与PELILLO等[23]利用HPLC-ELSD技术测定结果相近,小麦胚芽磷脂中PC、PI和PE的含量较高,且相对应的溶血性磷脂仅能检测到LPC和LPE,可判断PC和PE为小麦胚芽磷脂中主要的水解底物。样品中的sn-2溶血性磷脂含量相对较高,由于小麦胚芽中水解磷脂的主要磷脂酶为磷脂酶A2(PLA2),而PLA2会在sn-2位置特异性水解磷脂生成游离脂肪酸和溶血性磷脂,进一步被溶血磷脂酶和磷酸二酯酶分解生成甘油磷酰化合物(如GPC)、磷脂胆碱和游离胆碱(Cho)[36-37]。此外,脂肪酶和脂肪氧化酶也可水解和氧化磷脂。脂肪酶可水解PC生成LPC,也可进一步水解生成GPC[35, 38]。俞舜杰等[28]研究发现在蛋黄磷脂中添加脂肪氧合酶后,PC和PE的含量减少,对应的溶血性磷脂LPC和LPE的含量有所增加。酶活性、水分活度和反应温度等都是能够影响磷脂水解程度的因素[39]。由图1a和表5可看出,利用31P NMR测定出小麦胚芽中含磷化合物GPC和pi含量较高,摩尔浓度分别为0.75和1.00 μmol/g,两者都为非脂质含磷化合物,不属于磷脂[19, 33],但GPC是PC经过两步水解脱除脂肪酰基后得到的水解产物,检测GPC的含量可进一步探究磷脂水解变化情况。因此PC、PE、LPC、LPE和GPC的含量变化可作为探究小麦胚芽磷脂水解程度的指标。
除了31P NMR技术可以确定磷脂化合物的组成之外,1H NMR技术也可以应用于磷脂测定分析。1H NMR光谱中磷脂分析的化学位移信号主要位于3.16~4.25区域,如图1b所示,可分为1CH2(Δδ为3.95~4.25)、2CH2(Δδ为3.40~3.70)和N(CH3)3(Δδ为3.16~3.28)3个区域[24]。小麦胚芽样品的1H NMR光谱中可测定到PE、PC、LPC、GPC和Cho的信号,其中PE、PC、LPC和GPC都属于含胆碱化合物[40],Cho作为磷脂PC和PE的水解产物,则在图1b的光谱中能够检测到其特定峰。由于利用D2O水溶性溶剂测定得到的1H NMR光谱中化合物的化学位移较为相近,信号峰易重叠,分辨率较低,且报道其他磷脂组分化学位移的相关文献较少,因此只能对Cho进行定性定量分析,以及对含胆碱化合物PE、PC、LPC和GPC进行初步测定。而在31P NMR光谱中含磷化合物有且只有一个特征峰,因此31P NMR技术对磷脂组分进行定性定量分析更为合适,而1H NMR光谱可作为含胆碱化合物的补充说明。
2.2 小麦胚芽中性脂质的1H NMR分析
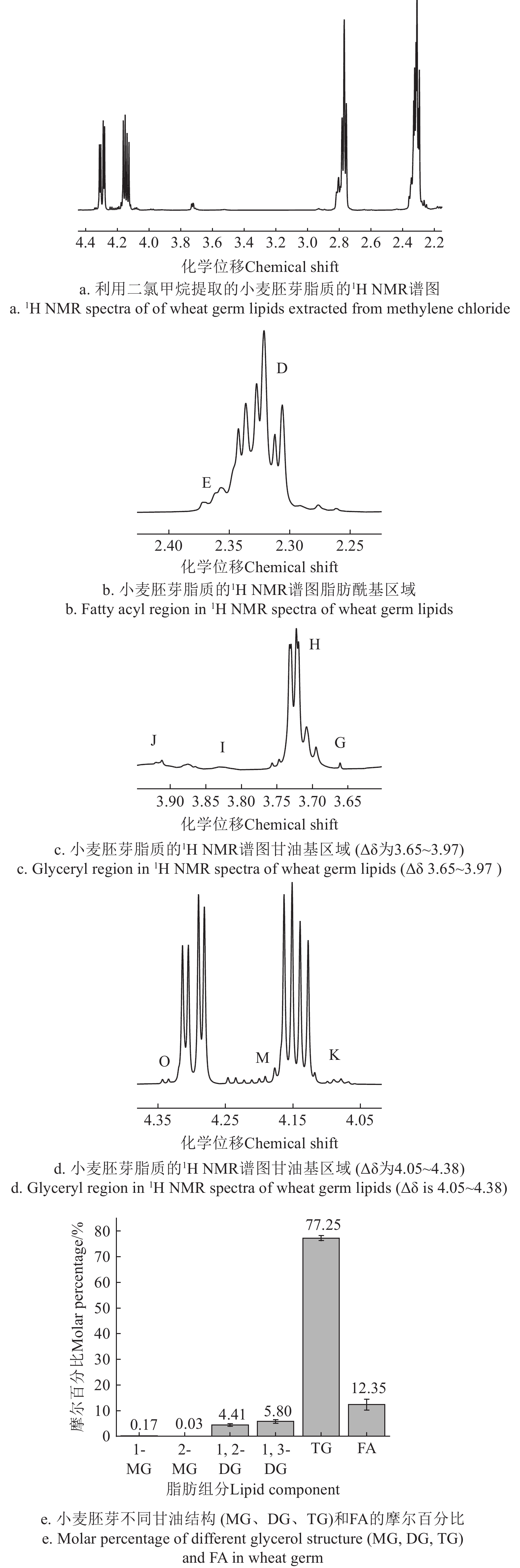
图2a为小麦胚芽的1H NMR光谱中脂肪酰基区域局部图(Δδ为2.20~4.40),图2b化学位移为2.26~2.42的脂肪酰基区域,图2c化学位移为3.65~3.97的甘油基区域,图2d化学位移为4.05~4.38的甘油基区域。小麦胚芽中所有甘油酯和游离脂肪酸的摩尔百分比如图2e所示,与文献[41]报道的结果相近。由图2e可知,小麦胚芽中游离脂肪酸(FA)占比12.35%,含量最高的甘油酯是TG,占比77.25%,其次1,3-甘油二酯(1,3-DG)占比5.80%,1,2-甘油二酯(1,2-DG)占比4.41%,1-甘油单酯(1-MG)占比0.17 %,含量最低的是2-甘油单酯(2-MG),占比0.03%。
TG被包裹在小麦胚芽脂质体中,当脂质体的磷脂膜被水解而破裂时,TG从中游离出来与小麦胚芽中的酶接触发生水解反应,TG逐渐被水解成产生甘油二酯(DG)、甘油单酯(MG)、游离脂肪酸(FA)和甘油。TG的sn-3位会被脂肪酶水解产生1,2-DG和相应的FA,1,2-DG的sn-1位被水解产生2-MG和相应的FA,2-MG可异构化生成1-MG,而1-MG和2-MG可继续被水解产生甘油和FA[12]。在整个水解过程中都会生成FA,因此小麦胚芽中FA的含量高于DG和MG。此外,甘油具有较高的极性,不溶于二氯甲烷,故在该试验中无法用1H NMR测定出小麦胚芽中甘油的含量。由于脂肪酶具有专一性[42],TG被脂肪酶水解不会生产1,3-DG,但从图2e中可看出1,3-DG的含量与1,2-DG相近,1,3-DG可能来源于1,2-DG的异构化和小麦胚芽中其他酶对甘油三酯的水解作用。
酶活性、含水率、温度和相对湿度等是影响TG水解程度的因素[43, 44]。通过1H NMR技术测定中性脂质的含量变化,可以分析脂质的水解程度[18]。李波[41]研究发现,随着贮藏时间的延长,小麦胚芽中TG的含量不断减少,FA的含量不断增加,DG和MG因酶的水解作用先产生而后又被水解,含量变化相对不稳定。因此TG和FA的含量变化更适合作为监测小麦胚芽脂质水解程度的指标。
2.3 小麦胚芽脂肪酰基的1H NMR分析
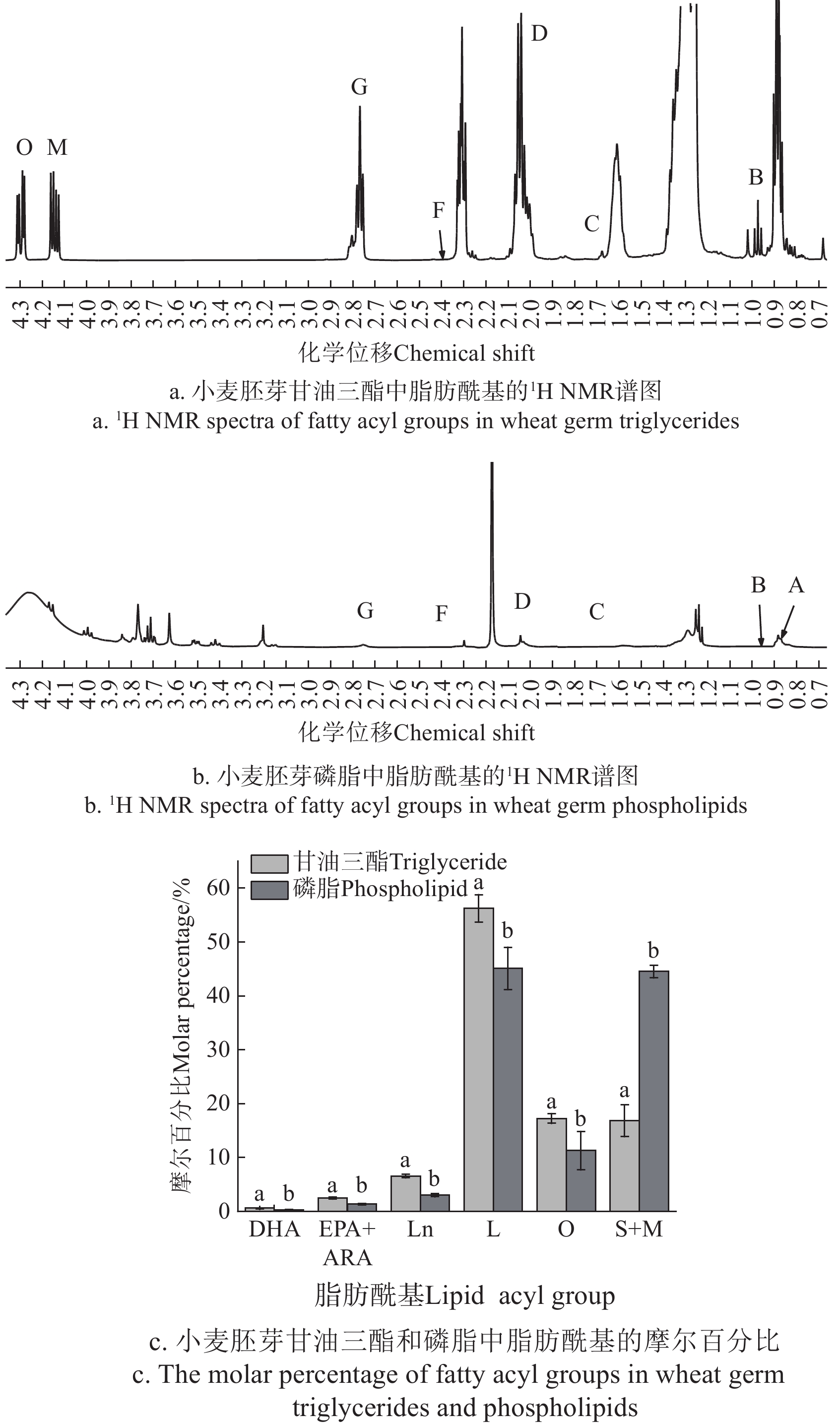
图3a和图3b分别为1H NMR光谱中小麦胚芽甘油三酯和磷脂的脂肪酰基区域图(Δδ为0.60~4.40),图3c为小麦胚芽磷脂和甘油三酯中主要酰基的摩尔百分比。
![]() 图 3 小麦胚芽甘油三酯和磷脂中脂肪酰基的NMR谱图和摩尔百分比注:谱图中信号峰上标与表2相对应;字母a和b代表了磷脂和甘油三酯脂肪酰基含量具有显著性差异(P < 0.05)。Figure 3. NMR spectra and molar percentages of fatty acyl groups in wheat germ triglycerides and phospholipidsNote: The superscript of the signal peak in the spectrum diagram corresponds to table 2. The letters a and b represent a significant difference between phospholipid and triglyceride fatty acyl content (P < 0.05).
图 3 小麦胚芽甘油三酯和磷脂中脂肪酰基的NMR谱图和摩尔百分比注:谱图中信号峰上标与表2相对应;字母a和b代表了磷脂和甘油三酯脂肪酰基含量具有显著性差异(P < 0.05)。Figure 3. NMR spectra and molar percentages of fatty acyl groups in wheat germ triglycerides and phospholipidsNote: The superscript of the signal peak in the spectrum diagram corresponds to table 2. The letters a and b represent a significant difference between phospholipid and triglyceride fatty acyl content (P < 0.05).如图3c所示,小麦胚芽中甘油三酯的脂肪酰基由0.65%二十二碳六烯酸(DHA)、2.47%二十碳五烯酸与花生四烯酸(EPA+ARA)、6.55%亚麻酸(Ln)、56.26%亚油酸(L)、17.24%油酸(O)以及16.83%饱和脂肪酸与修饰酰基(S+M)组成,磷脂的脂肪酰基由0.20% DHA、1.14% EPA与ARA、2.74% Ln、45.37% L、8.45% O以及45.36% S+M组成,小麦胚芽甘油三酯和磷脂中脂肪酰基成分具有显著性差异(P<0.05)。利用1H NMR技术测定的小麦胚芽甘油三酯脂肪酸组成与KUMAR等[45]利用甲酯化-气相色谱法测定结果相当。甘油三酯中不饱和脂肪酸在总脂肪酸中的占比超过80%,多不饱和脂肪酸在总脂肪酸中的占比超过65%,含量最高的多不饱和脂肪酸是L,其次是Ln。利用1H NMR技术测定的小麦胚芽中磷脂的脂肪酸组成中L和S+M的含量最高且相近,与NÉRON等[32]利用HPLC-ELSD技术测定小麦粉中磷脂脂肪酸组成相比,本研究中的亚油酸含量相对较少,饱和脂肪酸的含量相对较多,其原因可能是由于本试验中sn-2溶血性磷脂含量较高导致:大多数饱和脂肪酸位于溶血性磷脂的sn-1位上,而多不饱和脂肪酸(例如亚油酸)位于sn-2位上,因此sn-2溶血性磷脂分子内的多不饱和脂肪酸含量低[35, 46]。此结果也说明了本试验所用的小麦胚芽由于运输等原因导致水解程度增加、新鲜度下降。
3. 结 论
利用31P NMR(nuclear magnetic resonance)技术共检测到11种磷脂,分别为磷脂酰胆碱(PC)、磷脂酰肌醇(PI)、磷脂酰丝氨酸(PS)、磷脂酰乙醇胺(PE)、磷脂酰甘油(PG)、磷脂酸(PA)、sn-1溶血磷脂酰胆碱(sn-1 LPC)、sn-2溶血磷脂酰胆碱(sn-2LPC)、溶血磷脂酰乙醇胺(LPE)、sn-1溶血磷脂酰甘油(sn-1 LPG)和sn-2溶血磷脂酰甘油(sn-2 LPG),此外还能够检测出非脂质含磷化合物甘油磷脂酰胆碱(GPC)和无机磷酸盐(pi);小麦胚芽磷脂中含量最高的是PC,其摩尔浓度为0.42 μmol/g,摩尔分数为28.50 %,质量浓度为0.31 mg/g,质量分数为30.20 %。
1H NMR技术对小麦胚芽中性脂质和脂肪酸的组分含量进行测定,结果表明小麦胚芽中的甘油结构由甘油三酯、1,2-甘油二酯、1,3-甘油二酯、1-甘油单酯、2-甘油单酯、游离脂肪酸(FA)组成,占比分别为77.25%、4.41%、5.80%、0.17%、0.03%、12.35%;甘油三酯的脂肪酰基由二十二碳六烯酸(DHA)、二十碳五烯酸与花生四烯酸(EPA+ARA)、亚麻酸(Ln)、亚油酸(L)、油酸(O)、饱和脂肪酸与修饰酰基(S+M)组成,占比分别为0.65%、2.47%、6.55%、56.26%、17.24%、16.83%;磷脂的脂肪酰基由DHA、EPA+ARA、Ln、L、O和S+M组成,分别占比0.20%、1.14%、2.74%、45.37%、8.45%、43.56%。该研究表明,NMR能够有效且准确地对小麦胚芽脂质的组分进行定性定量;此外NMR技术能够检测到磷脂的水解产物(溶血性磷脂和GPC),以及甘油三酯的水解产物(甘油二酯、甘油单酯和游离脂肪酸)。
因此,利用NMR技术可以监测小麦胚芽的脂质变化,反映小麦胚芽脂质的水解程度,为研究其贮藏和加工特性提供技术参考,也为其他谷物类的脂质研究提供理论支持。
-
图 3 小麦胚芽甘油三酯和磷脂中脂肪酰基的NMR谱图和摩尔百分比
注:谱图中信号峰上标与表2相对应;字母a和b代表了磷脂和甘油三酯脂肪酰基含量具有显著性差异(P < 0.05)。
Figure 3. NMR spectra and molar percentages of fatty acyl groups in wheat germ triglycerides and phospholipids
Note: The superscript of the signal peak in the spectrum diagram corresponds to table 2. The letters a and b represent a significant difference between phospholipid and triglyceride fatty acyl content (P < 0.05).
表 1 小麦或小麦胚芽中含量最高的含磷化合物的分子信息
Table 1 Molecular information on the most abundant phosphorus-containing compounds in wheat or wheat germ
含磷化合物
Phosphorous-containing compound脂肪酰基组成
Fatty acyl groups composition化学分子式
Chemical formula相对分子质量
Relative molecular massPC 棕榈酸/亚油酸(16:0/18:2) C42H80NO8P 742.061 PI 棕榈酸/亚油酸(16:0/18:2) C43H79O13P 835.066 LPC 亚油酸(18:2) C26H50NO7P 519.660 PS 油酸/亚油酸(18:1/18:2) C42H76NO10P 786.027 PE 亚油酸/亚油酸(18:2/18:2) C41H74NO8P 740.002 GPC - C8H20NO6P 257.223 LPE 亚油酸(18:2) C23H44NO7P 477.572 PG 棕榈酸/亚油酸(16:0/18:2) C40H75O10P 746.991 LPG 棕榈酸(16:0) C22H45O9P 484.567 pi - PO43- 94.973 PA 亚油酸/亚油酸(18:2/18:2) C39H69O8P 696.947 注:PC为磷脂酰胆碱;PI为磷脂酰肌醇;LPC为溶血磷脂酰胆碱;PS为磷脂酰丝氨酸;PE为磷脂酰乙醇胺;GPC为甘油磷脂酰胆碱;LPE为溶血磷脂酰乙醇胺;PG为磷脂酰甘油;LPG为溶血磷脂酰甘油;pi为无机磷酸盐;PA为磷脂酸,下同。磷脂的相对分子质量参考文献[21-23];无机磷酸盐(pi)的相对分子质量依据磷酸根进行计算;甘油磷脂酰胆碱(GPC)和pi为非脂质含磷化合物,不属于磷脂,则不计算其质量分数。 Note: PC is phosphatidylcholine; PI is phosphatidylinositol; LPC is lysophosphatidylcholine; PS is phosphatidylserine; PE is phosphatidylethanolamine; GPC is glycerophosphocholine; LPE is lysophosphatidylethanolamine; PG is glycerophosphoglycerol; LPG is lysophosphatidylglycerol; pi is inorganic phosphate; PA is phospholipid acid,the same below. The relative molecular weight of phospholipids refers to relevant literature[21-23]; The relative molecular mass of inorganic phosphate (pi) is calculated according to phosphate group; Glycerol phosphatidylcholine (GPC) and pi are non-lipid phosphatide-containing compounds and do not belong to phospholipids, so their mass fractions are not calculated. 表 2 主要脂肪酰基在CDCl3中1H NMR信号的化学位移分配和多重峰种类
Table 2 Chemical shift assignments and multiplicities of the 1H NMR signals in CDCl3 of the main acyl groups
信号
Signal化学位移
Chemical shift δ峰型
Multiplicity官能团
Functional group组分
Compound参考文献
ReferenceA 0.83~0.93 m -CH3 饱和、单不饱和ω-9和/或ω-7、不饱和ω-6酰基 [28] B 0.97 t -CH3 不饱和ω-3酰基 [28] C 1.66~1.70 m -OCO-CH2-CH2- EPA和ARA酰基 [17] D 1.94~2.14 m -CH2-CH=CH- 除DHA外的脂肪酸烯丙基 [28] E 2.26~2.36 dt -OCO-CH2- 除DHA外的脂肪酰基 [18] F 2.36~2.42 m -OCO-CH2-CH2- DHA酰基 [28] G 2.76 t =HC-CH2-CH= 双不饱和ω-6酰基 [28] H 3.65 ddd ROCH2–CHOH–CH2OH 1-MG中的甘油基 [18] I 3.73 m ROCH2–CH(OR′)–CH2OH 1,2-DG中的甘油基 [18] J 3.84 m ROCH2–CH(OR′)–CH2OH 2-MG中的甘油基 [18] K 3.94 m ROCH2–CHOH–CH2OH 1-MG中的甘油基 [18] L 4.05~4.10 m ROCH2–CHOH–CH2OR′ 1,3-DG中的甘油基 [18, 29] M 4.14 dd -CH2OCOR TG中的甘油基 [17] N 4.18 ddd ROCH2–CHOH–CH2OH 1-MG中的甘油基 [18] O 4.30 dd -CH2OCOR TG中的甘油基 [17] P 4.32~4.38 ddd ROCH2–CH(OR')–CH2OH 1,2-DG中的甘油基 [18, 29] 注:信号与图2、图3中信号峰上标相对应。 Note: The signal corresponds to the superscript of the signal peak in figure 2 and figure 3. 表 3 不同甘油结构(MG、DG、TG)和FA的酰基的摩尔数(N)和摩尔百分比(AG)的计算式
Table 3 Calculation formula of the number of moles(N) and molar percentages of acyl groups (AG) supported on the different glyceride structures (MG, DG, TG) and fatty acid( FA)
脂肪组分
Lipid component摩尔数计算式
Molar number calculation formula摩尔百分比计算式
Molar percentage calculation formula1-MG N1-MG=PC⋅AK AG1-MG=N1-MGNT×100\text{%} 2-MG N2-MG=PC×AJ4 AG2-MG=N2-MGNT×100\text{%} 1,2-DG N1,2-DG=PC×(AH+I-2AK)2 AG1,2-DG=2N1,2-DGNT×100\text{%} 1,3-DG N1,3-DG=PC×(AL+M+N+O+P−2AK)5 AG1,3-DG=2N1,3-DGNT×100\text{%} TG NTG=PC×(2AO+P-AH+I+2AK)4 AGTG=3NTGNT×100\text{%} FA NFA=PC×AE+F-6NTG-4N1,2-DG−4N1,3-DG−2N1-MG−2N2-MG2 FA=NFANT×100\text{%} 注:1-MG为1-甘油单酯;2-MG为2-甘油单酯;1,2-DG为1,2-甘油二酯;1,3-DG为1,2-甘油二酯;TG为甘油三酯;FA为游离脂肪酸;Pc 是1H NMR波谱信号区与产生这些信号区的质子数之间的比例常数,15.84,计算方法为式(5);AK,AJ,AH+I,AL+M+N+O+P,AO+P和AE+F表示1H NMR波谱信号,与表2、图2信号峰上标相对应;该计算不考虑微量磷脂的影响[18]。 Note:1-MG is 1-monoacylglycerol; 2-MG is 2-monoacylglycerol; 1,2-DG is 1,2-diacylglycerol; 1,3-DG is 1,3-diacylglycerol; TG is triglyceride; FA is free fatty acid; Pc is the proportional constant between the signal region of 1H NMR spectrum and the number of protons generating these signal regions, Pc is 15.84, and the calculation method is shown in equation (5); AK, AJ, AH+I, AL+M+N+O+P, AO+P and AE+F represent 1H NMR spectral signals, corresponding to the superscript of signal peaks in table 2 and figure 2; This calculation does not take into account the effect of trace phospholipids[18]. 表 4 脂肪酰基的积分计算公式
Table 4 Integral calculation formula of fatty acyl groups
脂肪酰基
Lipid acyl group积分计算公式
Integral formulaLn ILn=AB3 EPA+ARA IEPA+ARA=AC2 DHA IDHA=AF4 L IL=AG2 O I(TG-O)=AD4-ILn-IL-IEPA+ARA I(PL-O)=AD4-(ILn-IDHA)2-IL-IEPA+ARA S+M IS+M=IFA-AD4-IDHA FA I(TG-FA)=AM+O4×3 I(PL-FA)=AA+B3 注:Ln为亚麻酸;EPA+ARA为二十碳五烯酸与花生四烯酸;DHA为二十二碳六烯酸;L为亚油酸;O为油酸;S+M为饱和脂肪酸与修饰酰基;FA为总脂肪酸;TG-O和TG-FA是指甘油三酯油酸和总脂肪酸,PL-O和PL-FA是指磷脂的油酸和总脂肪酸[17];AB,AC,AF,AG,AD,AM+O和AA+B表示1H NMR波谱信号,与表2、图3相对应。 Note: Ln is linolenic acid; EPA+ARA is eicosapentaenoic acid and arachidonic acid; DHA is docosahexaenoic acid; L is linoleic acid; O is oleic acid; S+M is saturated fatty acid and modified acyl group; FA is total fatty acids; TG-O and TG-FA refer to triglyceride oleic acid and total fatty acid, and PL-O and PL-FA refer to oleic acid and total fatty acid of phospholipids[17]; AB, AC, AF, AG, AD, AM+O and AA+B represent 1H NMR spectral signals, corresponding to the superscript of signal peaks in table 2 and figure 3. 表 5 利用31P NMR测定小麦胚芽含磷化合物的摩尔浓度、摩尔分数、质量浓度和质量分数
Table 5 Molar concentration, molar fraction, mass concentration and mass fraction of phosphorus-containing compounds in wheat germ were determined by 31P NMR
含磷化合物
Phosphorous-containing compound化学位移
Chemical shift (δ)摩尔浓度
Molar concentration/(μmol·g−1)摩尔分数
Molar fraction/%质量浓度
Mass concentration/(mg·g−1)质量分数
Mass fraction/%PC -0.87 0.42±0.03 28.50±1.33 0.31±0.03 30.20±1.46 PI -0.72 0.29±0.01 19.87±1.24 0.24±0.01 23.70±1.46 sn-1 LPC -0.58 0.04±0.01 2.48±0.83 0.02±0.01 1.84±0.64 PS -0.51 0.05±0.04 3.25±2.47 0.04±0.03 3.62±2.73 sn-2 LPC -0.40 0.11±0.01 7.64±0.49 0.06±0.00 5.67±0.39 PE -0.30 0.19±0.01 13.20±1.01 0.14±0.01 13.96±1.12 GPC -0.20 0.75±0.10 - 0.19±0.03 - LPE 0.13 0.06±0.02 4.37±1.56 0.03±0.01 2.98±1.08 PG 0.34 0.05±0.01 3.31±0.65 0.04±0.01 3.52±0.67 sn-1 LPG 0.84 0.03±0.01 2.01±0.63 0.01±0.00 1.39±0.44 sn-2 LPG 1.95 0.11±0.02 7.61±1.51 0.05±0.01 5.26±1.05 pi 2.65 1.00±0.14 - 0.09±0.01 - PA 3.34 0.16±0.02 10.63±1.09 0.11±0.01 10.58±1.08 TP - 1.46±0.06 - 1.02±0.04 - TPC - 3.21±0.22 - 1.31±0.06 - 注: sn-1 LPC为sn-1溶血磷脂酰胆碱; sn-2 LPC为sn-2溶血磷脂酰胆碱; sn-1 LPG为sn-1溶血磷脂酰甘油;sn-2 LPG为sn-2溶血磷脂酰甘油;TP为总磷脂;TPC为总含磷化合物。含磷化合物缩写与图1信号峰上标相对应,摩尔分数和质量分数指的是各磷脂在总磷脂中所占百分比,甘油磷脂酰胆碱(GPC)和无机磷酸盐(pi)不属于磷脂,则不计算其摩尔分数和质量分数。 Note: sn-1 LPC is sn-1 lysophosphatidylcholine; sn-2 LPC is sn-2 lysophosphatidylcholine; sn-1 LPG is sn-1 lysophosphatidylglycerol; sn-2 LPG is sn-2 lysophosphatidylglycerol; TP is total phospholipids; TPC is total phosphorus compounds. The abbreviations of phosphorus-containing compounds correspond to the superscript of the signal peak in figure 1, The molar fraction and mass fraction are the percentage of each phospholipid in the total phospholipid, glycerol phosphatidylcholine (GPC) and inorganic phosphate (pi) are not phospholipids, and their molar fraction and mass fraction are not calculated. -
[1] 徐斌,董英. 小麦胚芽的产业化开发现状与发展趋势[J]. 农业工程学报,2011,27(增刊2):341-345. XU Bin, DONG Ying. Present situation and trends of wheat germ industrialization developing[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2011, 27(Supp.2): 341-345. (in Chinese with English abstract)
[2] 徐斌,苗文娟,董英,等. 中国小麦胚芽资源分布及深加工相关品质[J]. 农业工程学报,2012,28(2):244-249. doi: 10.3969/j.issn.1002-6819.2012.02.042 XU Bin, MIAO Wenjuan, DONG Ying, et al. Resource distribution and processing quality of commercial wheat germ in China[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2012, 28(2): 244-249. (in Chinese with English abstract) doi: 10.3969/j.issn.1002-6819.2012.02.042
[3] MORUZZI G, VIVIANI R, SECHI A M, et al. Studies on compounds and individual lipids of wheat germ[J]. Journal of Food Science, 1969, 34(6): 581-584. doi: 10.1111/j.1365-2621.1969.tb12094.x
[4] XU B, HAN J H, ZHOU S L, et al. Quality characteristics of wheat germ oil obtained by innovative subcritical butane experimental equipment[J]. Journal of Food Process Engineering, 2015, 39: 79-87.
[5] HATZAKIS E. Nuclear magnetic resonance (NMR) spectroscopy in food science: a comprehensive review[J]. Comprehensive Reviews in Food Science and Food Safety, 2019, 18(1): 189-220. doi: 10.1111/1541-4337.12408
[6] 赵程祥,陈苏蒙,张宏达,等. 核磁共振波谱技术在食品质量与安全方面的应用[J]. 农产品质量与安全,2021(6):18-24 [7] RUNDLÖF T, MATHIASSON M, BEKIROGLU S, et al. Survey and qualification of internal standards for quantification by 1H NMR spectroscopy[J]. Journal of Pharmaceutical and Biomedical Analysis, 2010, 52(5): 645-651. doi: 10.1016/j.jpba.2010.02.007
[8] LI J B, VOSEGAARD T, GUO Z. Applications of nuclear magnetic resonance in lipid analyses: An emerging powerful tool for lipidomics studies[J]. Progress in Lipid Research, 2017, 68: 37-56. doi: 10.1016/j.plipres.2017.09.003
[9] YANG X Y, XIAO J S, WAN P, et al. The effect of lutein on the oxidation of egg yolk phospholipids in a liposome model[J]. Food Chemistry:X, 2023, 20: 100945. doi: 10.1016/j.fochx.2023.100945
[10] WAN P, ZHAO Z J, WANG Q Z, et al. Use of NMR, FTIR and GC-MS to monitor the oxidation products of phospholipids in bighead carp head during cooking process[J]. Journal of Food Composition and Analysis, 2024, 127: 105984. doi: 10.1016/j.jfca.2024.105984
[11] 叶婷婷,刘思佚,刘洁,等. NMR和GC-MS研究加热对奶油主要成分及香气化合物的影响[J]. 食品科学,2022,43(10):220-226. doi: 10.7506/spkx1002-6630-20210612-149 YE Tingting, LIU Siyi, LIU Jie, et al. Effect of thermal treatment on major ingredients and volatile compounds of cream studied by nuclear magnetic resonance spectroscopy and gas chromatography-mass spectrometry[J]. Food Science, 2022, 43(10): 220-226. (in Chinese with English abstract) doi: 10.7506/spkx1002-6630-20210612-149
[12] NIEVA-ECHEVARRÍA B, GOICOECHEA E, MANZANOS M J, et al. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures[J]. Food Research International, 2014, 66: 379-387. doi: 10.1016/j.foodres.2014.09.031
[13] 黄挺,王静羽,张伟,等. 近十年定量核磁共振法应用研究进展[J]. 化学试剂,2023,45(6):123-130. HUANG Ting, WANG Jingyu, ZHANG Wei, et al. Research progress in the application of quantitative nuclear magnetic resonance method in recent decade[J]. Chemical Reagents, 2023, 45(6): 123-130. (in Chinese with English abstract)
[14] 张娅妮,王国良,韩齐齐,等. 猕猴桃皮多酚提取及其对B16细胞黑色素的影响[J]. 农业工程学报,2022,38(7):317-325. doi: 10.11975/j.issn.1002-6819.2022.07.035 ZHANG Yani, WANG Guoliang, HAN Qiqi, et al. Extraction of the polyphenols from kiwifruit peel and its effect on melanin in B16 cells[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2022, 38(7): 317-325. (in Chinese with English abstract) doi: 10.11975/j.issn.1002-6819.2022.07.035
[15] 康乐天,王威皓,刘婷,等. 精氨酸对绵羊胴体品质、肉质特性及瘤胃细菌组成的影响[J]. 农业工程学报,2024,40(1):310-319. KANG Letian, WANG Weihao, LIU Ting, et al. Effects of arginine on carcass traits, meat quality characteristics and rumen bacterial composition of lambs[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2024, 40(1): 310-319. (in Chinese with English abstract)
[16] AHMMED M K, CARNE A, STEWART I, et al. Phosphorus-31 nuclear magnetic resonance (31P NMR) for quantitative measurements of phospholipids derived from natural products: effect of analysis conditions[J]. Food Science & Technology, 2021, 142: 110991.
[17] ZHAO Z J, WAN P, LIU J, et al. Monitoring of the oxidation process of egg yolk phospholipids at frying temperature by nuclear magnetic resonance[J]. Food Bioscience, 2023, 51: 102303. doi: 10.1016/j.fbio.2022.102303
[18] NIEVA-ECHEVARRÍA B, GOICOECHEA E, MANZANOS M J, et al. Usefulness of 1H NMR in assessing the extent of lipid digestion[J]. Food Chemistry, 2015, 179: 182-190. doi: 10.1016/j.foodchem.2015.01.104
[19] AHMMED M K, BUNGA S, STEWART I, et al. Simple and efficient one-pot extraction method for phospholipidomic profiling of total oil and lecithin by phosphorus-31 nuclear magnetic resonance measurements[J]. Journal of Agricultural and Food Chemistry, 2020, 68(48): 14286-14296. doi: 10.1021/acs.jafc.0c05803
[20] ALI A H, ZOU X Q, ABED S M, et al. Natural phospholipids: occurrence, biosynthesis, separation, identification, and beneficial health aspects[J]. Critical Reviews in Food Science and Nutrition, 2019, 59(2): 253-275. doi: 10.1080/10408398.2017.1363714
[21] GENG P, HARNLY J M, CHEN P. Differentiation of whole grain from refined wheat (T. aestivum) flour using lipid profile of wheat bran, germ, and endosperm with UHPLC-HRAM mass spectrometry[J]. Journal of Agricultural and Food Chemistry, 2015, 63(27): 6189-6211. doi: 10.1021/acs.jafc.5b01599
[22] LUCCI P, PACETTI D, CALZUOLA I, et al. Characterization of phospholipid molecular species and peptide molecules in wheat Sprout hydroalcoholic extract[J]. Journal of Agricultural and Food Chemistry, 2013, 61(47): 11453-11459. doi: 10.1021/jf4034392
[23] PELILLO M, FERIOLI F, IAFELICE G, et al. Characterisation of the phospholipid fraction of hulled and naked tetraploid and hexaploid wheats[J]. Journal of Cereal Science, 2010, 51(1): 120-126. doi: 10.1016/j.jcs.2009.11.002
[24] LOENING N M, CHAMBERLIN A M, ZEPEDA A G, et al. Quantification of phosphocholine and glycerophosphocholine with 31P edited 1H NMR spectroscopy[J]. NMR in Biomedicine, 2005, 18(7): 413-420. doi: 10.1002/nbm.973
[25] M A, P P, S M, et al. Absolute quantification of phospholipid metabolites in brain-tissue extracts by 1H NMR spectroscopy[J]. Journal of Magnetic Resonance. Series B, 1996, 113(2): 184-189. doi: 10.1006/jmrb.1996.0174
[26] COSTANTINI S, PARRILLO L, GUERRIERO E, et al. 1H-NMR metabolomic profiling of the crayfish Astacus leptodactylus subjected to polyphenol-enriched diets[J]. Aquaculture Nutrition, 2018, 24(1): 524-538. doi: 10.1111/anu.12585
[27] 楼乔明,徐华,王云鹏,等. 罗氏海盘车性腺脂质的微波提取及其成分分析[J]. 农业工程学报,2018,34(11):300-306. doi: 10.11975/j.issn.1002-6819.2018.11.038 LOU Qiaoming, XU Hua, WANG Yunpeng, et al. Microwave-assisted extraction of Asterias rollestoni gonad lipids and its component analysis[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2018, 34(11): 300-306. (in Chinese with English abstract) doi: 10.11975/j.issn.1002-6819.2018.11.038
[28] 俞舜杰,赵子建,万鹏,等. 利用核磁共振研究脂肪氧合酶酶促氧化蛋黄磷脂[J]. 食品科学,2022,43(22):121-128. doi: 10.7506/spkx1002-6630-20220114-132 YU Shunjie, ZHAO Zijian, WAN Peng, et al. Lipoxygenase-catalyzed oxidation of egg yolk phospholipids studied by nuclear magnetic resonance spectroscopy[J]. Food Science, 2022, 43(22): 121-128. (in Chinese with English abstract) doi: 10.7506/spkx1002-6630-20220114-132
[29] GOICOECHEA E, GUILLEN M D. Analysis of hydroperoxides, aldehydes and epoxides by 1H nuclear magnetic resonance in sunflower oil oxidized at 70 and 100℃[J]. Journal of Agricultural and Food Chemistry, 2010, 58(10): 6234-6245. doi: 10.1021/jf1005337
[30] REN C R, JIN J, ZHAO S W, et al. Phospholipid profiling, cholesterol, and tocopherols: comparison of sow milk fats from two lactation stages and five breeds[J]. Food Bioscience, 2022, 49: 101871. doi: 10.1016/j.fbio.2022.101871
[31] WEI W, CHENG J, YANG J, et al. Phospholipid composition and fat globule structure change during low temperature storage of human milk[J]. LWT-Food Science and Technology, 2021, 150: 112050. doi: 10.1016/j.lwt.2021.112050
[32] NÉRON S, AMRANI F E, POTUS J, et al. Separation and quantification by high-performance liquid chromatography with light scattering detection of the main wheat flour phospholipids during dough mixing in the presence of phospholipase[J]. Journal of Chromatography A, 2004, 1047(1): 77-83. doi: 10.1016/j.chroma.2004.06.105
[33] MACKENZIE A, VYSSOTSKI M, NEKRASOV E. Quantitative analysis of dairy phospholipids by 31P NMR[J]. Journal of the American Oil Chemists' Society, 2009, 86(8): 757-763. doi: 10.1007/s11746-009-1403-6
[34] SUGASINI D, SUBBAIAH P V, MIYAMOTO S. Rate of acyl migration in lysophosphatidylcholine (LPC) is dependent upon the nature of the acyl group. Greater stability of sn-2 docosahexaenoyl LPC compared to the more saturated LPC species[J]. PloS One, 2017, 12(11): e187826.
[35] VIKBJERG A F, MU H, XU X. Parameters affecting incorporation and by-product formation during the production of structured phospholipids by lipase-catalyzed acidolysis in solvent-free system[J]. Journal of Molecular Catalysis B:Enzymatic, 2005, 36(1/2/3/4/5/6): 14-21. doi: 10.1016/j.molcatb.2005.07.002
[36] WALTER A, KORTH U, HILGERT M, et al. Glycerophosphocholine is elevated in cerebrospinal fluid of Alzheimer patients[J]. Neurobiology of Aging, 2004, 25(10): 1299-1303. doi: 10.1016/j.neurobiolaging.2004.02.016
[37] 徐赢华,王国敬,李春,等. 酶法脱胶在植物油脂精炼中的应用进展[J]. 农业工程学报,2015,31(23):269-276. XU Yinghua, Wang Guojing , Li Chun, et al. Application of enzymatic degumming on vegetable oils refining[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2015, 31(23): 269-276. (in Chinese with English abstract)
[38] 高岩,卞永霞,庄姣,等. 冷藏过程中原料乳脂质代谢变化的靶向脂质组学分析[J]. 农业工程学报,2023,39(24):306-315. doi: 10.11975/j.issn.1002-6819.202308198 GAO Yan, BIAN Yongxia, ZHUANG Jiao, et al. Targeted lipidomic analysis of the changes in lipid metabolism of raw milk during refrigeration[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2023, 39(24): 306-315. (in Chinese with English abstract) doi: 10.11975/j.issn.1002-6819.202308198
[39] ANG X, CHEN H, XIANG J Q, et al. Preparation and functionality of lipase-catalysed structured phospholipid-A review[J]. Trends in Food Science & Technology, 2019, 88: 373-383.
[40] ZHAO Y Y, XIONG Y P, CURTIS J M. Measurement of phospholipids by hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry: The determination of choline containing compounds in foods[J]. Journal of Chromatography A, 2011, 1218(32): 5470-5479. doi: 10.1016/j.chroma.2011.06.025
[41] 李波. 小麦胚芽脂质快速酸败机制及稳定化研究[D]. 无锡:江南大学,2017. LI Bo. Study on Mechanism of Rapid Rancidity of Wheat Germ Lipids and its Stabilization Technology[D]. Wuxi: Jiangnan University, 2017. (in Chinese with English abstract)
[42] 徐斌. 微波辐射对小麦胚芽的稳定化作用机制及其应用研究[D]. 镇江:江苏大学,2011. XU Bin. Research on the Mechanism of Wheat Germ Stabilizationby Microwave Treatment and its Application[D]. Zhenjiang: Jiangsu University, 2011. (in Chinese with English abstract)
[43] 朱广飞,刘海,李卫,等. 油茶籽储藏品质变化规律及条件优化[J]. 农业工程学报,2020,36(2):301-311. doi: 10.11975/j.issn.1002-6819.2020.02.035 ZHU Guangfei, LIU Hai, LI Wei, et al. Change rule of storage quality and optimization of storage condition for Camellia oleifera seeds[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2020, 36(2): 301-311. (in Chinese with English abstract) doi: 10.11975/j.issn.1002-6819.2020.02.035
[44] 孙敏杰,迟玉杰,张明江. 提高蛋清粉起泡性能的工艺[J]. 农业工程学报,2008,24(11):274-278. SUN Minjie, CHI Yujiei, ZHANG Mingjiang. Technology for improving foaming properties of egg albumen powders[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2008, 24(11): 274-278. (in Chinese with English abstract)
[45] KUMAR G S, KRISHNA A G G. Studies on the nutraceuticals composition of wheat derived oils wheat bran oil and wheat germ oil[J]. Journal of Food Science and Technology, 2015, 52(2): 1145-1151. doi: 10.1007/s13197-013-1119-3
[46] OKUDAIRA M, INOUE A, SHUTO A, et al. Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS[J]. Journal of Lipid Research, 2014, 55(10): 2178-2192. doi: 10.1194/jlr.D048439
-
期刊类型引用(3)
1. 李敏,殷雄,刘雪婧,马世一,周延,种道彤,熊兵,李锟. 基于紫外光谱的油气两相流含气率检测研究. 光谱学与光谱分析. 2025(02): 522-531 .  百度学术
百度学术
2. 张京,赵泽瑄,赵艳茹,卜泓超,吴星宇. 基于Bi-GRU和空-谱信息融合的油菜菌核病侵染区域高光谱图像分割方法. 智慧农业(中英文). 2024(02): 40-48 .  百度学术
百度学术
3. 毛星,张欣,王宝佳,段玉林,李卫国,任妮. 长三角区域农业遥感应用:进展、挑战与展望. 中国农业信息. 2023(06): 37-48 .  百度学术
百度学术
其他类型引用(2)




 下载:
下载: