Quality characteristics of the extruded quinoa noodles with different grain colors
-
摘要:
为了探究不同粒色藜麦挤压面条品质特性的差异及机理,该研究对比分析了不同粒色藜麦粉的基础组分、糊化特性、挤压面条微观结构、蒸煮品质、活性成分及淀粉体外消化特性的差异。结果表明:白藜麦粉中淀粉含量最高,糊化后峰值黏度、最终黏度和回生值也最高。红、黑藜麦粉及挤压面条中的多酚、黄酮含量及抗氧化活性显著高于白藜麦粉和挤压面条。挤压后,淀粉结晶度均下降,白藜麦面条中淀粉的结晶度最高。红(2.25 min)、黑藜麦(2.75 min)面条的蒸煮时间短,但蒸煮品质显著低于白藜麦面条(P < 0.05)。扫描电镜显示白藜麦面条淀粉凝胶网络结构更加连续致密,导致其淀粉水解率和预计升糖指数显著低于红、黑藜麦面条(P < 0.05)。该研究为高品质藜麦面条的生产加工提供理论和技术参考。
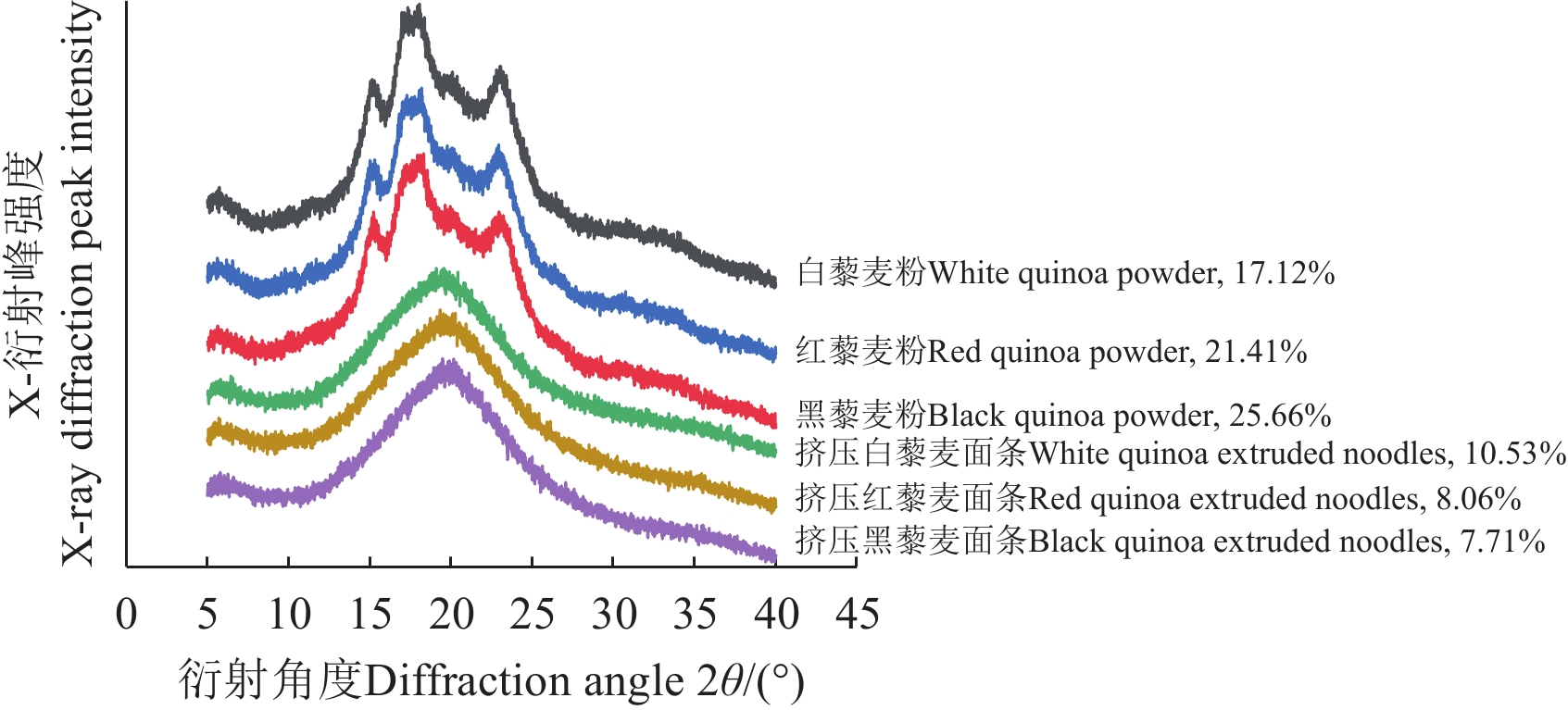
Abstract:Quinoa is one of the most popular ingredients in the creation of healthy and environmentally friendly foods, due to its rich nutrient composition, balanced amino acids, and various bioactive substances. Quinoa noodles can be prepared for green and healthy food, particularly for better taste and market prospects. However, the traditional processing of wheat noodles cannot fully meet the high quality of quinoa noodles at present, due to the lack of gluten. Among them, extrusion has been applied to prepare the whole quinoa noodles. The natural color of food can also be closely related to its nutritional value. Quinoa grains with different colors often vary in the content of starch, protein, fat, fiber, and active substances. This study aims to clarify the great influence on the cooking and nutritional quality of extruded noodles made with quinoa as raw materials. The mechanism of quality of extruded quinoa noodles with different colors was explored to compare the basic components, gelatinization of quinoa flours, the microstructure, cooking quality, active components, and starch digestion. Results showed that the white quinoa achieved the highest content of total starch and crude fiber, compared with the red and black quinoa powder. The content of crude fiber increased significantly with the deepening of the color of quinoa powder. Among the three types of quinoa powder, the red quinoa exhibited the highest levels of fat and protein content. In terms of gelatinization properties, the peak viscosity, final viscosity, and setback value of white quinoa powder were superior to those of red and black quinoa powder. The pasting temperature of white quinoa powder was lower than that of red and black quinoa powder. Furthermore, L* values significantly decreased after extrusion, whereas, a* value and b* values significantly increased (P < 0.05). Additionally, the red and black quinoa powder, along with their extruded noodles, exhibited higher levels of polyphenols, flavonoids, and antioxidant activities, compared with the white quinoa. X-ray diffraction revealed that the extrusion process caused the changes of starch crystal type from type A to V. There was a decrease of starch crystallinity in the white, red, and black quinoa noodles from 17.12%, 25.55%, and 21.41% to 10.53%, 8.06% and 7.71%, respectively. White quinoa noodles shared the highest crystallinity, due to the high starch content and high setback value of powder. The recrystallization of starch formed a relatively stable and orderly molecular structure, thus enhancing the crystallinity of starch in the extruded quinoa noodles. Although the cooking time of red and black quinoa extruded noodles was shorter than that of white ones, their overall cooking quality was notably inferior. The cooking loss of white quinoa noodles was 7.46%, which was significantly lower than that of red (10.12%) and black (9.16%). At the same time, the extruded white quinoa noodles also exhibited the highest hardness (30.71 N) and springiness (0.97). The extruded noodles relied primarily on the gelatinization and retrogradation of starch. The rearrangement of starch was dominated to form a stable gel structure after extrusion for the cooking quality of extruded quinoa noodles. Scanning electron microscope images confirmed that the gel network structure of white quinoa extruded noodles was denser and more complete, thereby leading to the lower cooking loss and superior texture, compared with the red and black quinoa extruded noodles. The in vitro starch digestibility showed that the white quinoa noodles contained the highest resistant starch content and the lowest predicted glycemic index of 68.56. In summary, the red and black quinoa presented a relatively higher content of active substances, but white quinoa was more suitable for the production of high-quality and low-GI extruded noodles. This finding can provide the theoretical and technical reference for the production and processing of high-quality quinoa noodles.
-
Keywords:
- quinoa /
- extruded noodles /
- cooking quality /
- nutritional characteristics /
- microstructure
-
0. 引 言
工业革命以来北半球地表O3浓度已经从10 nmol/mol升高到50 nmol/mol[1]。当前中国地表O3污染尤为严重[2],4—9月白天小时地表O3浓度超过40 nmol/mol的累积值(AOT40)约为30.9 μmol/(mol·h),并以每年10%的速率不断升高[3]。O3作为一种强氧化性的大气污染物,通过气孔进入植物叶片内部产生活性氧自由基,进而抑制叶片光合能力,改变同化物向地上组织的分配比例,最终导致作物减产[3-6]。现有的评估结果表明当前中国地表O3浓度水平已经导致小麦、水稻和玉米产量分别损失33%、23%和9%[7]。
O3浓度升高在减少作物产量的同时还可能影响农田生态系统的水分和能量交换,改变农田水热通量特征[8-9]。农田生态系统的能量主要来源于太阳辐射和地面反射的辐射。潜热是能量重要的消耗项[10],主要受农田微气象和作物生长的影响[11]。其中气孔导度控制了作物蒸腾速率,极大地影响了农田生态系统潜热通量[12-13]。O3浓度升高对气孔导度具有重要影响[14-15],一方面可以通过抑制光合能力增加细胞间隙CO2浓度,从而降低气孔导度[16-17]。另一方面O3诱导植物乙烯排放降低了气孔对脱落酸响应的敏感性[18],从而导致气孔调节失灵,无法调节气孔导度响应环境变化。因此O3浓度升高改变了作物冠层蒸腾,进而影响了农田生态系统水热通量特征。
农田开放式O3浓度升高平台(O3-FACE)通过对农田微气象和土壤热通量的连续观测,应用能量平衡法可以分析O3浓度升高对潜热通量、感热通量和土壤热通量分配的影响[19]。当前能量平衡法已经被应用在美国的农田开放式气体浓度升高平台(SoyFACE),基于该方法研究了不同O3浓度对大豆农田水热通量的变化特征[20]。结果表明O3浓度升高增加了冠层温度与空气温度差,进而提高了感热通量,显著降低潜热通量。因此O3浓度升高导致叶片气孔关闭和降低冠层蒸腾,最终减少了农田生态系统的水分消耗[21]。O3浓度升高关闭气孔导度限制了叶片光合能力,最终减少作物籽粒产量。在SoyFACE也发现大豆籽粒产量随O3暴露剂量升高而显著降低。但产量的损失大于水分消耗的减少,显著降低了田间水分利用效率。分析O3浓度升高对农田水热通量和产量的影响,对提高水分利用效率具有重要意义。
小麦是中国第二大粮食作物,相比于其他作物,其对地表O3浓度升高更为敏感[7]。利用O3-FACE平台开展4a的研究发现,O3浓度升高50%导致小麦产量平均损失19%。但小麦品种O3抗性存在显著差异,其中扬辐麦2号、烟农19和扬麦16较敏感,而扬麦15和嘉兴002则表现出较强的O3抗性[22-25]。FENG等[7]通过建立亚洲区域的O3剂量响应关系,评估发现相比于工业革命前的O3浓度水平,2017—2019年地表O3污染降低了中国小麦产量约28.2%~36.9%,长江三角洲地区水稻年平均减产约为2.445×109 t,约占实际产量的9.1%[26]。研究还显示, O3在降低产量的同时还可能改变了农田生态系统的蒸散和能量分配。然而较少有研究关注O3浓度升高对麦田水热通量和田间水分利用效率的影响,这为制定合理的小麦灌溉策略带来了不确定性。
利用不同育成年份的小麦品种研究表明,新育成的小麦品种对O3更敏感[27]。当前江淮地区的主推品种已经更新,2007—2010年在O3-FACE平台测试的扬辐麦2号、烟农19和嘉兴002等品种已不再被推广使用[22-25]。过去10多年时间内缺乏利用O3-FACE研究O3浓度升高对小麦影响的试验。亟待了解中国江淮地区当前主推小麦品种的O3敏感性,研究O3浓度升高对当前小麦产量和麦田水热通量的影响。为此本研究利用位于江苏省扬州市的O3-FACE系统平台,选用当前江淮地区主推品种“农麦88”,通过对麦田小气候和土壤热通量进行连续监测,结合能量平衡法定量研究O3浓度升高对当前品种下麦田水热通量和水分利用效率的影响,以期为O3浓度升高下保障小麦产量和优化麦田用水管理提供田间水分利用效率的理论依据。
1. 材料与方法
1.1 试验区概况
试验于江苏省扬州市江都区南京信息工程大学绿色农业研究与示范基地(119°43′E, 32°25′N)进行。试验站地处长江三角洲,属亚热带季风气候,是中国小麦主产区之一。2009—2018年该地区年均气温、年降水总量和年日照小时总数分别为16.2 ℃、1131.3 mm和1936.1 h。
1.2 试验设计
试验在研究组建设的完全开放式O3浓度升高平台(O3-FACE)中进行。本研究共设置两个O3浓度处理,即环境O3浓度(AA)和1.5倍环境O3浓度(E-O3),两个O3浓度各设置4个重复,共计8个直径为14 m的正八边形FACE圈。在E-O3的FACE圈边缘安装8根长度6 m带有直径0.5 mm微孔的PPR管道。管道随着作物的生长升高,始终位于作物冠层上方20 cm左右的高度。高浓度O3由O3发生器电离纯氧产生,与压缩空气混合后通过管道传送到每个样地,通过PPR管上的微孔向圈中心喷射O3混合气体,在每个圈中心利用O3分析仪(Thermo 49i)对O3浓度进行实时监测。与环境O3浓度进行实时对比后,自动调节O3发生器功率和流量。保持E-O3圈内O3浓度始终为AA圈内O3浓度的1.5倍。各圈之间间隔大于50 m,以避免各个圈O3浓度相互干扰。本试验从2021年3月4日开始O3处理,晴朗天气每天熏蒸10 h(8:00—18:00),阴雨天不熏蒸,同年6月1日停止熏蒸。O3处理期间AA和E-O3白天10 h平均O3浓度分别为(41.37 ± 0.63)和(61.22 ± 2.57) nmol/mol,AOT40分别为(9.37 ± 0.55)和(25.43 ± 2.02) μmol/(mol·h)。
本试验在AA圈和E-O3圈内各设置面积9 m2的正方形试验小区。以当前在江苏地区播种面积最大的农麦88作为供试品种,于2020年11月11日播种,播种密度为160 株/m2。田间N施肥量为225 kg/hm2,其中基肥∶分蘖肥∶拔节肥∶穗肥为 5∶1∶2∶2,日常农艺措施与大田一致。小麦在2021年3月16日开始拔节,4月4日孕穗期,4月13日抽穗,4月19日开花,4月25日进入灌浆期,5月16日乳熟,6月2日收获。O3浓度升高对小麦生育期无显著影响。
1.3 农田辐射平衡观测系统
分别在AA和E-O3处理安装了农田辐射平衡监测系统,每个处理设置3个重复。每个小区的辐射平衡系统观测面积约为9 m2。本研究从2021年3月13日—2021年6月1日(小麦拔节期-成熟期)对每个样地的净辐射(Rn,W/m2)、空气温度(Ta,℃)、冠层温度(Tc,℃)、空气湿度(RH,%)、土壤热通量(G0,W/m2)、土壤温度(Tsoil,℃)、土壤含水率(Wsoil,%)和风速(WS,m/s)进行观测。
净辐射传感器(NR Lite2)和风速风向传感器(034B)安装在距地面2 m的高度。在距离地面1.5 m处空气温湿度传感器(083E)和植物冠层红外温度传感器(S1-1H1),红外温度传感器与水平面成 45°角, 其监测的小麦冠层面积约为3.8 m2。在地下2 cm处分别埋设土壤温度热电偶探头(Omega T,USA)以监测土壤温度,在5 cm处埋设土壤热通量板(HFP01)用以监测土壤热通量。土壤水分探头(CS616)垂直埋设,监测0~30 cm平均土壤含水率。所有传感器连接到 CR1000 数据采集器(Campbell Scientific Inc,USA)上,每隔10 s采集 1 次数据,每隔10 min计算并记录 1 次平均值。
1.4 水热通量观测
本研究采用叶片温度与空气温度差计算感热通量,通过能量平衡方法[19]计算冬小麦的潜热通量(λET,W/m2),计算如下:
λET=Rn−H−G0 (1) 式中Rn为净辐射,W/m2; H为感热通量,W/m2;G0为地表土壤热通量,W/m2。蒸散利用潜热通量换算得到[28]。
感热通量(H)利用冠层温度和空气温度差值进行计算[29]:
H=ρacρTc−Tara (2) 式中ρa为空气密度,1.23 kg/m3;cρ是为空气的热容量,1018.32 J/kg℃;Tc和Ta分别为冠层和空气温度,℃;ra为空气动力阻力[30],s/m。
地表土壤热通量是通过使用0.05 m处的土壤热通量板测量土壤热通量来确定的,再加上对板上方热量储存变化的估计[10],计算如下:
G0=Gs+dTsdtHs(ρbCs+ρwCwWsoil) (3) 式中Gs为0.05 m深度处的土壤热通量,W/m2;dTs为地下0.02 m土壤温度的变化,K;t为计算时间间隔,600 s;Hs为土壤热通量板埋的深度,0.05 m;ρb为土壤容重,1661 kg/m3;ρw为水密度,1000 kg/m3;Cs和Cw分别为土壤和水的比热,为840和4190 J/(kg·K);Wsoil为0~0.3 m深度的平均土壤体积含水率,%。
1.5 SPAD测定
采用SPAD-502便携式叶绿素测定仪(Minolta Camera Co., Ltd., Chiyoda City, Japan)测定SPAD,从2021年3月21日—2021年5月22日每隔7~15 d,每个样地随机选取3株代表平均长势的小麦并对其顶部新展开的叶片进行SPAD值测定,每片顶部叶片测3个位点取平均值,共计测定15次SPAD。
1.6 籽粒产量和产量组成测定
各试验小区于成熟期在每个小区内随机取20个穗,自然风干后手工脱粒并称质量,而后百粒板计算籽粒数量。计算穗粒数和千粒质量。在未取样区域划定2个1 m2样方作为测产区域,手工收获麦穗并记录麦穗个数,自然风干后脱粒并称质量。
1.7 数据分析
使用Excel对观测数据进行整理,利用Shapiro-Wilk 和 Levene分别检验数据正态性和方差齐性,并利用R软件4.1.0(http://www.rproject.org/)进行统计分析。O3浓度升高对净辐射、土壤热通量、感热通量和潜热通量等的影响采用lme函数中的线性混合效应模型进行方差分析,采用 Tukey’s HSD法进行多重比较分析。
2. 结果与分析
2.1 O3浓度升高对小麦SPAD的影响
从图1可知,SPAD随小麦的生长呈先缓慢上升后迅速下降的趋势。小麦叶片SPAD最大值出现在灌浆期,在AA和E-O3分别为58.3和57.3。O3浓度升高对SPAD最大值无显著影响。从5月16日起小麦开始衰老,其SPAD值开始快速降低。O3浓度升高显著降低了5月22日的叶片SPAD。相比于AA,E-O3的SPAD平均降低了11.5。
2.2 O3浓度升高下麦田水热通量季节变化特征
为了研究O3浓度升高对麦田水热通量的影响,分析了O3处理期间日间(8:00—18:00)麦田净辐射、土壤热通量、感热通量和潜热通量的日变化特征。表1和图2分别展示了AA和E-O3处理的麦田水热通量季节变化特征和不同生育期内日均值。
表 1 环境空气(AA)和O3浓度升高(E-O3)下不同生育期内麦田感热通量H、潜热通量λET、净辐射Rn、土壤热通量G0日均值和峰值Table 1. Daily means and peak values of sensible heat flux H, latent heat flux λET, net radiation Rn, soil heat flux G0 during different growth periods for AA and E-O3 (W·m−2)项目
Item时期
StagesH λET Rn G0 AA E-O3 AA E-O3 AA E-O3 AA E-O3 日均值
Daily meansJo-Bo 72.87±32.68a 59.51±11.58a 96.58±36.27a 109.02±8.24a 208.67±3.26a 210.23±5.35a 39.64±4.74a 41.94±2.03a Bo- He 92.30±46.88a 68.52±12.79a 170.31±47.13a 190.50±6.14a 300.48±4.52a 303.62±9.04a 38.03±3.78a 44.60±2.48a He- MA 89.97±30.71a 72.84±11.73a 175.55±30.68a 184.60±7.20a 298.01±4.51a 297.94±7.79a 32.64±6.28a 40.51±3.63a MA- GF 51.74±23.83a 34.75±10.25a 131.09±25.81a 145.96±8.05a 216.61±1.43a 219.46±6.17a 33.78±6.31a 38.75±4.39a GF - MR 17.53±14.26a 10.36±5.13a 234.71±15.03a 238.59±5.32a 289.95±4.17a 290.75±5.73a 37.69±6.21a 41.88±4.69a MR- Ma 89.29±22.72a 96.95±9.73a 163.89±15.93a 156.74±13.29b 288.88±6.49a 290.18±4.51a 35.79±5.16a 36.63±4.17a Ma-WG 65.01±26.12a 56.30±5.20a 163.86±25.86a 170.44±2.31a 265.79±4.15a 267.26±5.84a 37.07±4.00a 40.63±2.96a 峰值
Peak valuesJo-Bo 142.42±56.84a 120.91±20.70a 130.94±62.29a 149.13±16.38a 342.72±4.63a 345.66±7.61a 69.37±6.55a 75.62±2.86a Bo- He 185.74±86.90a 141.24±24.91a 245.51±82.89a 273.63±11.83a 483.84±6.91a 487.39±13.39a 52.59±4.10a 72.52±1.65b He- MA 157.13±50.86a 132.33±20.88a 253.62±47.92a 258.95±13.90a 456.70±7.04a 456.12±10.04a 45.95±4.80a 64.84±4.96b MA- GF 81.59±35.41a 60.64±16.31a 191.10±37.11a 198.93±14.73a 318.42±5.20a 317.94±7.30a 45.73±6.51a 58.38±7.25b GF - MR 39.29±22.84a 30.77±8.88a 340.14±17.46a 349.51±11.32a 450.71±5.82a 450.55±9.52a 71.21±7.56a 70.27±6.74a MR- Ma 151.89±28.49a 171.33±19.22a 230.05±21.83a 208.67±25.58b 446.54±9.49a 444.27±5.03a 64.60±7.05a 64.27±5.03a Ma-WG 120.24±41.95a 108.20±8.96a 233.41±40.98a 239.89±4.80a 416.73±6.18a 417.26±8.98a 63.06±4.23a 69.16±3.53a 注:日均值和峰值分别表示O3熏蒸后8:00—18:00和12:00—13:00 峰值10 min数据的平均值(平均值±标准差)。Jo、Bo、He、MA、GF、MR、Ma、WG分别代表拔节期、孕穗期、抽穗期、开花期、灌浆期、乳熟期、成熟期和全生育期。不同小写字母表示差异显著(P<0.05)。 Note: Daily means and peak values indicate the average value (mean ± standard deviation) of the 10 min data during the day from 8:00 to 18:00 and 12:00 to 13:00 (Peak values) after ozone fumigation. Jo, Bo, He, MA, GF, MR, Ma, WG indicate jointing, booting, heading, anthesis, grain-filling, milk ripening, maturity, whole growing season, respectively. Different lowercase letters in the table indicate significant differences (P<0.05). 在小麦生长季节内,感热通量总体呈先逐步降低而后快速升高的趋势,AA和E-O3的平均值分别为56.01和56.30 W/m2(表1)。O3浓度升高对不同生育期阶段的感热通量日均值无显著影响(图2a和表1)。
潜热通量代表植物蒸腾和土壤水分蒸发所消耗的能量总和。在生长季尺度上,潜热通量呈先上升后下降的趋势,在灌浆期达到最大值,随后在乳熟期呈下降的趋势,整个生长季消耗的能量分别为163.86和170.44 W/m2。O3浓度升高对潜热通量日均值无显著影响(图2b),但是显著降低了乳熟–成熟期的潜热通量。对其他生育期的潜热通量均无影响(表1)。
净辐射呈缓慢升高的季节性变化趋势,在整生长季总能量分别为265.79和267.26 W/m2。在不同生育期内O3浓度升高对净辐射无显著影响(表1)。仅在极少数天数上有显著影响,O3浓度升高显著降低了3月20日和5月16日的净辐射,但升高了4月27日和5月20日的净辐射。O3浓度升高对净辐射具有显著影响的日期,均为净辐射较低的阴天,且无明显规律(图2c)。
土壤热通量主要受太阳辐射和小麦冠层覆盖遮挡的影响。在季节尺度上呈缓慢降低而后升高趋势,最低值出现在抽穗–开花期,整个生长季分别为37.07和40.63 W/m2。本研究中水热通量从小麦拔节初期开始进行观测,此时小麦已基本覆盖地表,太阳辐射难以直接到达土壤。因此土壤热通量在观测期间较低。O3浓度升高对不同生育期的麦田土壤热通量均无显著影响(表1)。在季节尺度上,O3浓度升高仅对4月4日、4月9日和5月1日的土壤热通量有显著影响,对其他天无显著影响(图2 d)。
2.3 O3浓度升高下麦田水热通量日变化特征
为了分析O3浓度升高对小麦不同生育期水热通量日变化影响特征,计算了拔节–孕穗、孕穗–抽穗、抽穗–开花、开花–灌浆、灌浆–乳熟和乳熟–成熟内每天对应时间段的感热通量、潜热通量、净辐射、土壤热通量的10 min平均值。提取正午(12:00—13:00)的水热通量,分析O3浓度升高对不同生育期内正午通量的影响。
感热通量在AA和E-O3分别占净辐射能量的24.50%和21.07%,在日尺度氮上升后下降,在12:00左右达到峰值。在06:00和18:00左右分别完成正负值转变(图3)。感热通量正午值随生育期呈升高–降低–升高的过程。O3浓度升高对不同生育期内感热通量日变化和峰值均无显著影响(图3,表1)。潜热通量分别占全生育期能量支出的61.65%和63.77%。潜热通量的日变化趋势表现为,05:30左右转向正值,18:30左右接近0,夜间近地面大气温度低于露点温度是导致有部分时间潜热通量小于0 的主要原因(图3)。在生育期内潜热通量呈先上升后下降的趋势。O3浓度升高导致乳熟-成熟期的正午峰值显著降低了21.68 W/m2,但对其他生育期并没有产生显著影响(表1)。
净辐射是小麦农田主要的能量输入来源,其主要受到太阳辐射影响,随太阳日出和日落变化。从06:00开始升高,在12:30左右达到峰值随后下降;其夜间净辐射出现负值主要是地表向大气散热导致。O3浓度升高对各生育期内的净辐射正午峰值均无显著影响(表1)。
土壤热通量分别占净辐射输入能量的13.95%和15.20%,其日变化特征与净辐射一致。06:00到17:00为正值,其余时间为负值,并在12:00左右达到最大值。夜间表层土壤向大气散热是导致土壤热通量小于0 的主要原因(图3)。O3浓度升高显著升高了孕穗–抽穗、抽穗–开花和开花–灌浆期的土壤热通量正午峰值(表1)。
2.4 O3浓度升高对小麦产量、产量组成和水分利用效率的影响
由表2可知小麦籽粒产量在AA和E-O3分别为648.41和637.88 g/m2;穗密度分别为306.38和314.38 个/m2;千粒质量分别为60.37和59.01 g/千粒;穗粒数分别为51.84和53.98。O3浓度升高对小麦籽粒产量及产量组成(穗密度、千粒质量、穗粒数)均无显著影响(P>0.05)。试验处理期间麦田蒸散量在AA为198.49 mm,E-O3为210.82 mm;田间水分利用效率在AA为3.27 g/(m2 mm),E-O3为3.02 g/(m2 mm)。O3浓度升高对麦田蒸散量和水分利用效率均无显著影响(P>0.05)。
表 2 O3浓度升高对小麦穗密度和产量的影响Table 2. Effects of elevated ozone on ear density and grain yield of wheat处理
Treatment穗密度
Ear density/
(个·m−2)籽粒产量
Grain yield/
(g·m−2)千粒质量
Thousand grain
weight/g穗粒数
Grain number
per ear蒸散
Evapotranspiration/
mm水分利用效率
Water use efficiency/
(g·m−2 mm−1)AA 306.38±36.48 648.41±58.40 60.37±1.02 51.84±3.02 198.49±31.75 3.27±0.35 E-O3 314.38±27.81 637.88±47.34 59.01±3.12 53.98±1.63 210.82±3.46 3.02±0.25 3. 讨 论
农田生态系统蒸散研究为农田灌排决策提供了重要科学依据[31],对优化农田用水管理提供水分利用效率具有重要意义。但现有研究尚未关注O3浓度升高对麦田水热通量的影响特征。为此本研究利用完全开放式O3浓度升高平台,通过系统观测农田小气候特征,利用能量平衡法分析了O3浓度升高对麦田水热通量的影响,结合小麦籽粒产量进一步探讨了田间水分利用效率对O3浓度升高的响应。结果表明O3浓度升高降低了灌浆后期小麦叶片的叶绿素含量(图1)。但对麦田水热通量无显著影响,仅降低了乳熟到成熟期的潜热通量(表1)。同时O3浓度升高也未影响该小麦品种的籽粒产量、产量组成和水分利用效率(表2)。
O3是具有植物毒性的大气污染物,通过气孔进入植物体内产生活性氧[32-33],但是当O3吸收通量超过了植物自身解毒能力,细胞膜将被氧化,破坏叶绿体结构引起叶绿素降解[34],进而抑制植物叶片光合速率,加速叶片衰老[35-37]。本研究也发现O3浓度升高显著降低了灌浆后期的小麦叶片叶绿素含量,加速了叶片衰老(图1),但对其他生育期内叶绿素含量无显著影响。FENG等[38]利用FACE研究也发现小麦在生殖生长期对O3更敏感,对营养生长期小麦叶片无任何影响。这主要是由于O3对叶片的损失具有累积效应。孙陶芳[22]利用FACE平台也发现O3浓度升高显著降低了4种小麦品种花后20~25 d叶片的叶绿素含量,其中扬辐麦2号和烟农19较为敏感。而扬麦16和扬麦15较为抗,在花后25 d才显著降低。本研究采用的是江淮地区当前主推的小麦品种农麦88,其叶绿素在花后32 d才显著降低。因此O3浓度升高对农麦88的叶绿素含量具有损失作用,但是其O3抗性远远高于过去研究的小麦品种。
O3浓度升高降低叶绿素含量,进而抑制叶片光合速率[32,36]。小麦叶片为了降低无效水分消耗,会减少气孔开合,进而降低了冠层蒸腾,最终影响麦田潜热通量和水热通量分配比例。本研究对不同生育期的水热通量进行分析,结果表明O3浓度升高对净辐射和感热通量无显著影响,但显著降低了乳熟-成熟期的潜热通量日均值和正午峰值(表1、表2)。灌浆后期O3浓度升高显著降低了小麦叶绿素含量、光合速率和气孔导度,进而降低该阶段的麦田潜热通量。VANLOOCKE等[20]利用相同的观测方法在美国Soybean FACE上研究了O3浓度升高对大豆田水热通量的影响,结果也发现O3浓度升高对净辐射和土壤热通量没有显著差异,显著增加了大豆冠层温度和感热通量,降低了潜热通量和田间蒸散量。田间水分消耗的减少还显著提高了表层土壤的水分含量[19]。而本研究中O3浓度升高对试验期间麦田平均水热通量均无显著影响。可能的原因是当前栽培的小麦品种具有较高的O3抗性,仅在灌浆后期的极个别天内降低了田间蒸散,对生长季节内的水热通量不足以造成显著性差异。而大豆作为O3敏感性品种,O3浓度升高对其叶片光合速率和气孔导度均具有显著影响,进而改变了大豆田水热通量分配特征。这是本研究结果和过去研究结论不同的关键。
作物田间水分利用效率反应了作物生产过程中用水效率的关键指标,提高水分利用效率是农田水管理的关键目标。本研究表明O3浓度升高对麦田用水效率无显著影响,对麦田蒸散、籽粒产量和产量组成均无显著影响(表2)。美国Soybean FACE平台的研究表明O3浓度升高导致大豆产量降低了约64%,蒸散降低了约26%,因此导致水分利用效率降低了50%,这与有关研究形成了鲜明的对比。过去研究均表明O3浓度升高显著降低了小麦籽粒产量[39],孙陶芳[22]利用FACE平台发现O3浓度升高1.5倍导致扬辐麦 2 号、扬麦 15、烟农 19 和扬麦 16产量分别减少 25.6%、15.5%、18.7%和19.5%。XU等[40]整合全球的小麦试验结果分析也发现中国小麦对O3敏感性远高于欧洲和美国。相比于玉米和水稻,小麦是对O3最为敏感性的作物[7]。本研究发现O3浓度升高对小麦产量和产量组成均无显著影响,作为对O3最为敏感的千粒质量也未响应O3浓度升高。本研究O3浓度升高了约50%与过去的FACE研究相类似,且环境O3浓度高于之前的研究。2011年之后中国一直没有基于FACE研究O3浓度升高对小麦的影响,缺乏对当前使用新品种O3敏感性的了解。本研究是时隔10年,再一次利用FACE平台对当前江淮地区主栽小麦品种O3响应的研究。本研究的叶绿素、水热通量和产量的结果均表明农麦88具有较强的O3抗性。大面积种植该品种有助于减缓O3浓度升高对小麦产量和麦田蒸散的影响。
4. 结 论
本研究利用完全开放式O3浓度升高系统研究了O3浓度升高对麦田水热通量的影响。研究结果表明:1)相比于10年前的FACE试验,当前江淮地区主推的农麦88具有极好的O3抗性。2)O3浓度升高降低了乳熟–成熟期小麦叶片叶绿素含量和潜热通量。但是小麦生长季内麦田水热通量、籽粒产量、产量组成和水分利用效率对O3浓度升高无显著影响。因此需要重新审视利用过去研究数据评估O3污染对小麦产量和麦田蒸散的影响。在O3浓度不断升高背景下,种植抗逆性强的小麦品种可能是实现粮食稳产和优化田间水分利用效率的有效途径。未来需针对其他主栽品种开展多年试验,明确O3浓度升高对中国小麦产量和麦田水热通量的影响,为O3浓度升高背景下中国麦田合理灌溉和区域水循环过程模拟提供理论依据。
-
图 1 挤压前后不同粒色藜麦粉多酚含量和黄酮含量变化
注:大写字母不同表示同种处理不同粒色品种藜麦差异显著(P < 0.05),小写字母不同表示同种粒色藜麦不同处理差异显著(P < 0.05),下同。
Figure 1. Changes of polyphenol content and flavonoid content of quinoa powder with different grain colors before and after extrusion
Note: Different capital letters indicate significant difference when compared between quinoa with different colors of the same treatment (P < 0.05), and different lowercase letters indicate significant difference when compared between different treatments of quinoa with the same color (P < 0.05), the same below.
图 5 不同粒色藜麦挤压面条淀粉水解率和淀粉百分比
注:RDS为快消化淀粉,SDS为慢消化淀粉,RS为抗性淀粉;字母不同表示样品间差异显著(P < 0.05)。
Figure 5. The starch hydrolysis curve and starch percentage of quinoa extruded noodles with different grain colors
Note: RDS was rapid digestible starch, SDS was slow digestible starch, RS was resistant starch; Different lowercase letters indicate significant difference between the samples (P < 0.05).
表 1 不同粒色藜麦粉的组分质量分数(湿基)
Table 1 Basic ingredients content of raw quinoa powder with different grain colors (wet base)
% 样品
Sample灰分
Ash脂肪
Lipid蛋白质
Protein总淀粉
Total starch粗纤维
Crude fibre水分
Moisture白藜麦
White quinoa3.02±0.07b 6.66±0.02b 13.55±0.02a 58.49±0.39c 5.08±0.06a 9.05±0.03a 红藜麦
Red quinoa2.64±0.02a 6.91±0.04c 14.22±0.17b 53.50±0.20a 6.57±0.03b 10.84±0.09b 黑藜麦
Black quinoa2.60±0.17a 6.45±0.35a 13.62±0.09a 55.01±0.56b 7.87±0.07c 11.72±0.01c 注:同一列的字母不同表示样品间差异显著(P < 0.05),下同。 Note: Different lowercase letters indicate significant difference between the samples (P < 0.05), the same below. 表 2 不同粒色藜麦原粉的糊化参数
Table 2 Gelatinization parameters of raw quinoa powder with different grain colors
样品
Sample峰值黏度
Peak viscosity /(mPa·s)谷值黏度
Hot paste viscosity /(mPa·s)崩解值
Breakdown value /(mPa·s)最终黏度
Final viscosity /(mPa·s)回生值
Setback value /(mPa·s)成糊温度
Pasting temperature /oC白藜麦
White quinoa1806±15c 1774 ±15c32±10a 2432 ±41c658±30b 68±0a 红藜麦
Red quinoa1282 ±38a1107 ±13a175±26b 1412 ±33a305±22a 71±1a 黑藜麦
Black quinoa1399 ±32b1385 ±30b14±4a 1642 ±51b257±21a 79±5b 表 3 挤压前后不同粒色藜麦粉色差的变化
Table 3 Changes of color properties of quinoa powder with different grain colors before and after extrusion
处理
Treatment样品
Sample亮度(L*)
Brightness红度(a*)
Redness黄度(b*)
Yellowness挤压前
Before extrusion白藜麦
White quinoa91.14±0.07Bc 0.22±0.02Aa 11.94±0.17Aa 红藜麦
Red quinoa73.79±0.33Bb 4.30±0.11Ac 14.7±0.05Ab 黑藜麦
Black quinoa71.94±0.02Ba 3.00±0.02Ab 11.90±0.06Aa 挤压后
After extrusion白藜麦
White quinoa83.63±0.11Ac 0.30±0.01Ba 17.83±0.17Bb 红藜麦
Red quinoa59.04±0.15BAb 8.94±0.15Bc 19.38±0.31Bc 黑藜麦
Black quinoa56.76±0.20Aa 5.89±0.10Bb 15.63±0.24Ba 注:小写字母不同表示同种处理下不同粒色品种藜麦差异显著(P < 0.05),大写字母不同表示同种粒色藜麦不同处理差异显著(P < 0.05)。 Note: Different lowercase letters indicate significant difference when compared between quinoa with different colors of the same treatment (P < 0.05), and different capital letters indicate significant difference when compared between different treatments of quinoa with the same color (P < 0.05). 表 4 不同粒色藜麦挤压面条蒸煮特性和质构特性
Table 4 Cooking quality and textural properties of quinoa extruded noodles with different grain colors
样品
Sample蒸煮时间
Cooking time/
min蒸煮损失
Cooking loss/%硬度
Hardness /N弹性
Springiness内聚性
Cohesiveness咀嚼度
Chewiness白藜麦
White quinoa3.50±0c 7.46±0.32a 30.71±0.54c 0.97±0.01b 0.74±0.01c 1768 ±10c红藜麦
Red quinoa2.25±0a 10.12±0.20c 18.82±0.27a 0.89±0.02a 0.60±0a 1073 ±9a黑藜麦
Black quinoa2.75±0b 9.16±0.19b 27.64±0.13b 0.90±0.11a 0.62±0b 1636 ±20b表 5 不同粒色藜麦挤压面条的计算平衡浓度、酶解速率、水解指数、预测血糖指数
Table 5 The calculated equilibrium concentration, enzymatic hydrolysis speed rate, hydrolysis index, predicted glycemic index of quinoa extruded noodles with different grain colors
藜麦颜色
Grain colors平衡浓度
Calculated
equilibrium
concentration酶解速率
Enzymatic
hydrolysis
speed rate水解指数
Hydrolysis
index预测血糖指数
Predicted
glycemic index白藜麦
White quinoa45.36±0.23a 0.01±0a 52.54±0.29a 68.56±0.60a 红藜麦
Red quinoa56.98±0.55b 0.01±0a 69.00±0.70c 77.59±0.93c 黑藜麦
Black quinoa45.95±0.11a 0.01±0a 58.19±0.40b 71.66±0.85b -
[1] 付丽红,李晓斌. 基于岭脊分析的藜麦淀粉提取及糊化特性研究[J]. 农业工程学报,2016,32(18):299-306. doi: 10.11975/j.issn.1002-6819.2016.18.041 FU Lihong, LI Xiaobin. Extraction and gelatinization characteristics of Chenopodium quinoa Willd starch based on ridge analysis[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(18): 299-306. (in Chinese with English abstract) doi: 10.11975/j.issn.1002-6819.2016.18.041
[2] 陈茜,王振兴,孙健,等. 藜麦的营养成分、生物活性及加工利用[J]. 生物加工过程,2023,21(3):292-300. doi: 10.3969/j.issn.1672-3678.2023.03.008 CHEN Xi, WANG Zhenxing, SUN Jian, et al. Research progress on nutritional components, functional activities, and processing and utilization of quinoa[J]. Chinese Journal of Bioprocess Engineering, 2023, 21(3): 292-300. (in Chinese with English abstract) doi: 10.3969/j.issn.1672-3678.2023.03.008
[3] SUN X, MENG L, TANG X. Retrogradation behavior of extruded whole buckwheat noodles: An innovative water pre-cooling retrogradation treatment[J]. Journal of Cereal Science, 2021, 99: 103234. doi: 10.1016/j.jcs.2021.103234
[4] XU X, MENG L, GAO C, et al. Construction of starch-sodium alginate interpenetrating polymer network and its effects on structure, cooking quality and in vitro starch digestibility of extruded whole buckwheat noodles[J]. Food Hydrocolloids, 2023, 143: 108876. doi: 10.1016/j.foodhyd.2023.108876
[5] SUN X, YU C, FU M, et al. Extruded whole buckwheat noodles: effects of processing variables on the degree of starch gelatinization, changes of nutritional components, cooking characteristics and in vitro starch digestibility[J]. Food & Function, 2019, 10(10): 6362-6373.
[6] 王润,党斌,杨希娟,等. 青稞低GI挤压面条制作工艺优化及营养与抗氧化活性分析[J]. 中国粮油学报,2019,34(6):37-44. doi: 10.3969/j.issn.1003-0174.2019.06.008 WANG Run, DANG Bin, YANG Xijuan, et al. Processing technology optimization and nutrition and antioxidant activity analysis of highland barley low glycemic index extruded noodles[J]. Journal of the Chinese Cereals and Oils Association, 2019, 34(6): 37-44. (in Chinese with English abstract) doi: 10.3969/j.issn.1003-0174.2019.06.008
[7] 张鑫,任元元,孟资宽,等. 低GI藜麦面条挤压工艺及体外消化特性研究[J]. 食品与发酵科技,2021,57(4):57-62. doi: 10.3969/j.issn.1674-506X.2021.04.008 ZHANG Xin, REN Yuanyuan, MENG Zikuan, et al. Study on extrusion technology and in vitro digestion characteristics of low GI quinoa noodles[J]. Food and Fermentation Sciences & Technology, 2021, 57(4): 57-62. (in Chinese with English abstract) doi: 10.3969/j.issn.1674-506X.2021.04.008
[8] TANG Y, LI X, ZHANG B, et al. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes[J]. Food Chemistry, 2015, 166: 380-388. doi: 10.1016/j.foodchem.2014.06.018
[9] QIAN G, LI X, ZHANG H, et al. Metabolomics analysis reveals the accumulation patterns of flavonoids and phenolic acids in quinoa (Chenopodium quinoa Willd. ) grains of different colors[J]. Food Chemistry: X, 2023, 17: 100594. doi: 10.1016/j.fochx.2023.100594
[10] 洪佳敏,林宝妹,张帅,等. 6种杂粮营养成分分析及评价[J]. 食品安全质量检测学报,2019,10(18):6254-6260. HONG Jiamin, LIN Baomei, ZHANG Shuai, et al. Analysis and evaluation of nutritional components in 6 kinds of minor cereals[J]. Journal of Food Safety and Quality, 2019, 10(18): 6254-6260. (in Chinese with English abstract)
[11] HAN Y, CHI J, ZHANG M, et al. Characterization of saponins and phenolic compounds: Antioxidant activity and inhibitory effects on α-glucosidase in different varieties of colored quinoa (Chenopodium quinoa Willd)[J]. Bioscience, Biotechnology, and Biochemistry, 2019, 83(11): 2128-2139. doi: 10.1080/09168451.2019.1638756
[12] WANG X, LAO X, BAO Y, et al. Effect of whole quinoa flour substitution on the texture and in vitro starch digestibility of wheat bread[J]. Food Hydrocolloids, 2021, 119: 106840. doi: 10.1016/j.foodhyd.2021.106840
[13] 张林华,张红玉,谢凯文,等. 探究后处理工艺对藜麦挤压面条品质的影响[J/OL]. 中国粮油学报:1-12 [2024-05-23]. https: //doi. org/10.20048/j. cnki. issn. 1003-0174.000257. ZHANG Linhua, ZHANG Hongyu, XIE Kaiwen, et al. Investigation of the influence of the post-treatment techniques of quinoa extruded noodles on their quality[J/OL]. Journal of the Chinese Cereals and Oils Association: 1-12. https://doi.org/10.20048/j.cnki.issn.1003-0174.000257. (in Chinese with English abstract)
[14] BAKAR M F A, MOHAMED M, RAHMATB A, et al. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus)[J]. Food Chemistry, 2009, 113(2): 479-483. doi: 10.1016/j.foodchem.2008.07.081
[15] RE R. Antioxidant activity applying an improved ABTS radical cation decolorization assay[J]. Free Radical Biology and Medicine, 1999, 26(9-10): 1231-1237. doi: 10.1016/S0891-5849(98)00315-3
[16] XU X, GAO C, XU J, et al. Hydration and plasticization effects of maltodextrin on the structure and cooking quality of extruded whole buckwheat noodles[J]. Food Chemistry, 2022, 374: 131613. doi: 10.1016/j.foodchem.2021.131613
[17] GOH R, GAO J, ANANINGSIH V K, et al. Green tea catechins reduced the glycaemic potential of bread: An in vitro digestibility study[J]. Food Chemistry, 2015, 180: 203-210. doi: 10.1016/j.foodchem.2015.02.054
[18] WOOLNOUGH J W, BIRD A R, MONRO J A, et al. The effect of a brief salivary α-amylase exposure during chewing on subsequent in vitro starch digestion curve profiles[J]. International Journal of Molecular Sciences, 2010, 11(8): 2780-2790. doi: 10.3390/ijms11082780
[19] BHAT F M, RIAR C S. Effect of composition, granular morphology and crystalline structure on the pasting, textural, thermal and sensory characteristics of traditional rice cultivars[J]. Food Chemistry, 2019, 280: 303-309. doi: 10.1016/j.foodchem.2018.12.064
[20] 张宏,林向阳,朱榕璧,等. 淀粉类制品加工特性影响因素的研究[J]. 农产品加工(学刊),2008(11):16-18. ZHANG Hong, LIN Xiangyang, ZHU Rongbi, et al. Study on the influencing factors for processing of characteristics of starch products[J]. The Processing of Agricultural Products, 2008(11): 16-18. (in Chinese with English abstract)
[21] 李进才,朱凯莉,刘梦杰,等. 藜麦营养组分与加工品质的相关性分析[J]. 天津大学学报(自然科学与工程技术版),2022,55(6):655-663. LI Jincai, ZHU Kaili, LIU Mengjie, et al. Correlation analysis between the nutritional component and processing quality of quinoa[J]. Journal of Tianjin University: Science and Technology, 2022, 55(6): 655-663. (in Chinese with English abstract)
[22] 霍金杰,王可心,岳喜庆,等. 槲皮素对挤压大米淀粉结构及功能特性的影响[J/OL]. 农业工程学报:1-8[2024-05-23]. http://kns.cnki.net/kcms/detail/11.2047.S.20240514.1416.002.html. HUO Jinjie, WANG Kexin, YUE Xiqing, et al. Effects of quercetin on the structure and functional properties of extrusion rice starch[J/OL]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE): 1-8[2024-05-23]. http://kns.cnki.net/kcms/detail/11.2047.S.20240514.1416.002.html. (in Chinese with English abstract)
[23] 姚哲,张辉,彭金龙,等. 不同品种大米营养组分与糊化、酶解特性的相关性分析[J]. 食品与发酵工业,2022,48(13):173-180. YAO Zhe, ZHANG Hui, PENG Jinlong, et al. Correlation analysis between nutrient components, pasting properties and enzymatic hydrolysis properties of different varieties of rice[J]. Food and Fermentation Industries, 2022, 48(13): 173-180. (in Chinese with English abstract)
[24] 吴迪,葛飞,马红,等. 不同磨粉方式对青稞全粉理化特性的影响[J]. 中国粮油学报,2022,37(3):59-67. doi: 10.3969/j.issn.1003-0174.2022.03.010 WU Di, GE Fei, MA Hong, et al. Effects of different milling methods on physicochemical properties of highland barley whole flour[J]. Journal of the Chinese Cereals and Oils Association, 2022, 37(3): 59-67. (in Chinese with English abstract) doi: 10.3969/j.issn.1003-0174.2022.03.010
[25] 王莉,王鹏,于小帅,等. 挤压体系中可得然胶对小麦淀粉回生特性和冻融稳定性的影响[J]. 农业工程学报,2024,40(1):331-338. WANG Li, WANG Peng, YU Xiaoshuai, et al. Effects of curdlan on wheat starch retrogradation and freeze-thaw stability in the extrusion system[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE): 2024, 40(1): 331-338. (in Chinese with English abstract)
[26] JIA B, YAO Y, LIU J, et al. Physical properties and in vitro starch digestibility of noodles substituted with tartary buckwheat flour[J]. Starch - Stä rke, 2019, 71(5/6): 1800314.
[27] BRAHMA S, WEIER S A, ROSE D J. Effects of selected extrusion parameters on physicochemical properties and in vitro starch digestibility and β-glucan extractability of whole grain oats[J]. Journal of Cereal Science, 2016, 70: 85-90. doi: 10.1016/j.jcs.2016.05.001
[28] JAFARI M, KOOCHEKI A, MILANI E. Effect of extrusion cooking on chemical structure, morphology, crystallinity and thermal properties of sorghum flour extrudates[J]. Journal of Cereal Science, 2017, 75: 324-331. doi: 10.1016/j.jcs.2017.05.005
[29] 赵广河,张瑞芬,苏东晓,等. 全谷物酚类物质及其抗氧化活性研究进展[J]. 中国食品学报,2017,17(8):183-196. ZHAO Guanghe, ZHANG Ruifen, SU Dongxiao, et al. The advances on polyphenols in whole grains and its antioxidant activity[J]. Journal of Chinese Institute of Food Science and Technology, 2017, 17(8): 183-196. (in Chinese with English abstract)
[30] SHARMA P, GUJRAL H S, SINGH B. Antioxidant activity of barley as affected by extrusion cooking[J]. Food Chemistry, 2012, 131(4): 1406-1413. doi: 10.1016/j.foodchem.2011.10.009
[31] ALVAREZ-JUBETE L, WIJNGAARD H, ARENDT E K, et al. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking[J]. Food Chemistry, 2010, 119(2): 770-778. doi: 10.1016/j.foodchem.2009.07.032
[32] 杜春婷,党斌,杨希娟,等. 不同加工方式对藜麦淀粉结构与功能特性的影响[J]. 中国粮油学报,2022,37(4):54-61. doi: 10.3969/j.issn.1003-0174.2022.04.009 DU Chunting, DANG Bin, YANG Xijuan, et al. Effects of different processing methods on structural and functional properties of quinoa starch[J]. Journal of Chinese Institute of Food Science and Technology, 2022, 37(4): 54-61. (in Chinese with English abstract) doi: 10.3969/j.issn.1003-0174.2022.04.009
[33] VELáSQUEZ-BARRETO F F, MIñANO H A, ALVAREZ-RAMIREZ J, et al. Structural, functional, and chemical properties of small starch granules: Andean quinoa and kiwicha[J]. Food Hydrocolloids, 2021, 120: 106883. doi: 10.1016/j.foodhyd.2021.106883
[34] 王磊鑫,吴娜娜,吕莹果,等. 挤压蒸煮加工米糠可溶和不溶膳食纤维对米淀粉性质的影响及其相互作用分析[J]. 食品科学,2022,43(16):107-113. doi: 10.7506/spkx1002-6630-20210918-231 WANG Leixin, WU Nana, LV Yingguo, et al. Effect of soluble and insoluble dietary fibers from extrusion cooked rice bran on the properties of rice starch and their interactions[J]. Food Science, 2022, 43(16): 107-113. (in Chinese with English abstract) doi: 10.7506/spkx1002-6630-20210918-231
[35] XIA R, FU M, WANG Z, et al. Effects of frozen storage on the quality characteristics of frozen whole buckwheat extruded noodles[J]. Food Chemistry, 2023, 429: 136856. doi: 10.1016/j.foodchem.2023.136856
[36] 任立焕. 马铃薯面条加工工艺的研究[D]. 天津:天津科技大学,2017:23-24. REN Lihuan. The Processing Technology Research of Potato Noodles[D]. Tianjin: Tianjin University of Science and Technology, 2017: 23-24. (in Chinese with English abstract)
[37] 陶春生,陈存社,王克俭. 挤压改性麦麸膳食纤维对面条品质的影响[J]. 食品科技,2017,42(9):132-136 TAO Chunsheng, CHEN Cunshe, WANG Kejian. Effects of extrusion modification of wheat bran dietary fiber on quality of noodle[J]. Food Science and Technology, 2017, 42(9): 132-136. (in Chinese with English abstract)
[38] 卜宇,陈秋桂,牛倩文,等. 淀粉老化调控对燕麦全粉挤压面条蒸煮品质的影响[J]. 麦类作物学报,2017,37(10):1327-1333. doi: 10.7606/j.issn.1009-1041.2017.10.08 BU Yu, CHEN Qiugui, NIU Qianwen, et al. Effect of starch retrogradation on the cooking quality of whole oat flour extrusion noodles[J]. Journal of Triticeae Crops, 2017, 37(10): 1327-1333. (in Chinese with English abstract) doi: 10.7606/j.issn.1009-1041.2017.10.08
[39] DENG N, DENG Z, TANG C, et al. Formation, structure and properties of the starch-polyphenol inclusion complex: A review[J]. Trends in Food Science & Technology, 2021, 112: 667-675.
[40] 张天学. 热处理对青稞淀粉结构和性质的影响[D]. 广州:华南理工大学,2017:58-83. ZHANG Tianxue. Effect of Heat Treatment on Structure and Properties of Highland Barley Starch[D]. Guangzhou: South China University of Technology, 2017: 58-83. (in Chinese with English abstract)
[41] PENG M, YIN L, DONG J, et al. Physicochemical characteristics and in vitro digestibility of starches from colored quinoa (Chenopodium quinoa) varieties[J]. Journal of Food Science, 2022(5): 87.
[42] XU M, WU Y, HOU G G, et al. Evaluation of different tea extracts on dough, textural, and functional properties of dry Chinese white salted noodle[J]. Lwt-Food Science and Technology, 2019, 101: 456-462. doi: 10.1016/j.lwt.2018.11.066





 下载:
下载: